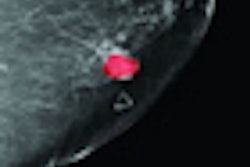
Imaging Diagnostic Systems (IDSI) has received notification from the U.S. Food and Drug Administration (FDA) requesting more information for a 510(k) application it submitted for its CT laser mammography (CTLM) imaging device.
IDSI is working with its FDA regulatory advisors to respond to the request, according to the firm. In the meantime, the application has been placed on hold until the information has been received, IDSI said.