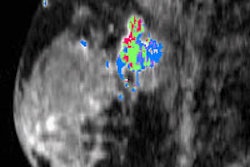
Computer-aided detection (CAD) developer Parascript has received U.S. Food and Drug Administration (FDA) premarket approval (PMA) for version 6.1 of its AccuDetect software.
AccuDetect is indicated for use in screening mammography to identify areas suspicious for breast cancer for radiologist review after completing an initial read, according to the vendor. The software is approved for use with digital mammography systems manufactured by GE Healthcare and Philips Healthcare.
AccuDetect is currently being used commercially in Spain, France, Germany, and Austria, Parascript said.