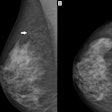
The nipple aspirate test is not a substitute for a mammogram, according to the U.S. Food and Drug Administration (FDA).
Some companies are promoting the test as a substitute for a mammogram, the FDA said in a statement. The nipple aspirate test uses a breast pump to collect fluid to screen for abnormal and potentially cancerous cells, and it's being touted as an easier and less painful option than a mammogram.
However, there is no clinical evidence to support the claim that the nipple aspirate test can be used as an alternative to mammography to screen for and diagnose breast cancer, according to the FDA. Women should not forgo a mammogram and have the test instead, the agency advised.
In February, the FDA issued a warning letter to Atossa Genetics informing the company that its ForeCyte breast health test's labeling was false or misleading. The agency asked the firm to take prompt action to correct the violations addressed in the warning letter. In October, Atossa initiated a voluntary recall to remove the test from the market, the FDA said.