AuntMinnie.com presents the 16th in a series of columns on the practice of ultrasound from Dr. Jason Birnholz, one of the pioneers of the modality.
Fellow Ultrasounder,
I do not think there is any medical area where there is more rampant public confusion than breast cancer screening, which for most purposes means any of a number of forms of x-ray mammography. This began about a half-century ago when waves of women's groups -- who originally promoted palpation with talcum powder and later with soap suds -- began agitating for mammography.
 Dr. Jason Birnholz.
Dr. Jason Birnholz.When concerns about radiation exposure arose, low-dose techniques seemed to satisfy the undeterred public and private drive for x-ray mammography. Even the public attention to "dense breasts" seems to have accepted the primacy of x-ray mammography and focused on the reporting of findings rather than addressing root issues. Amidst much controversy, some researchers have concluded that screening mammography has not altered all-cause mortality, which has implications throughout the medical imaging community.
Screening
Ultrasound people all know about screening. Think about typical obstetrical exams. They always have elements of screening for congenital anomalies, premature labor risk, and signs of supply line deprivation. The majority of exams are normal.
Expectant moms nearly always want ultrasound exams, and for them the failure of screening -- that is to say, reassurance about the likelihood that everything is fine -- is of greater value than any kind of risk identification.
The elements of a screening trial include the following:
- A grave condition with a sufficiently high prevalence in the population
- A reasonably effective marker for identifying or excluding that condition
- An effective form of therapy
- A population that wants to be tested; this type of screening is referred to as "active case-finding"
There is an implicit assumption that detection will be at an early stage of the disease, when treatment will most likely be successful and complete. It is essential that all of the testing be uniform and standardized; screening is a diagnostic triage procedure.
Screening is risk identification, not diagnosis. These poles do occasionally converge, though, particularly when we search for a condition that has a specific molecular or genetic marker. There are elements of screening in every medical office visit, such as patient history, blood pressure, an electrocardiogram, or a blood metabolic panel.
In many of these instances, a probabilistic or relative risk level can be identified -- as with a prostate-specific antigen (PSA) or cardiac panel -- in which there are large population statistics, an automated test procedure with a continuous numerical value, and a well-defined clinical end point. A risk level is a probabilistic estimate; it has a distribution with some type of spread. It is not an exact figure and, as such, is most suited to populations, not individuals.
Let's say your goal is to detect trisomy 21 antenatally, as early in pregnancy as possible, in a high percentage of pregnant women. We want to do this as accurately as possible, which might mean tolerating a low level of false positives to avoid missing any cases.
The solution in the 1970s was to combine a group of individually insensitive maternal blood markers, and in the 1990s, it was to use nuchal translucency (NT), initially in combination with the previously identified blood tests. Obviously neither of these is ideal or even that good, but they are better than nothing.
Now, we have cell-free DNA. This test is diagnostic, completely reliable, automated, objective, safe, and applicable in the first trimester. It should be offered to everyone who wants prenatal screening without discrimination by age or any other ethnic or socioeconomic concern.
The age criterion came about from a 1970s calculation of when the age-related risk of aneuploidy exceeded the risk of an amniocentesis. Amnios became much safer over time, but third-party payors never seemed eager to drop the cutoff age to anywhere near the recalculated level. Whether you can make simple comparisons between a procedure risk and a prevalence statistic or not, a safe age plainly does not apply for a blood test in a pregnant woman.
Fully replacing ultrasound-determined NT istimely because there seem to be a lot of "protocols" being suggested for preserving this test and reserving cell-free DNA, none of which seem to involve free NT surveys. That does not mean that ultrasound should be abandoned; obviously, there are so many more fetal concerns that cannot be addressed in any other way. But it's time to drop it for screening for the most common forms of aneuploidy.
Medicine is inherently conservative. As we move toward evidence-based practice criteria, it becomes increasingly important to discard screening procedures when better forms become available, despite our familiarity with what has worked for us some of the time in the recent past. This is an issue that has plagued breast screening since its onset.
Mass screening for tuberculosis
Issues about screening can arise when it is a standard of care applied to the entire population or when it is an unstandardized procedure provided as a paid service by private institutions or practitioners on demand or discriminately. The prototype mass screening program relying on imaging was miniature chest films for tuberculosis (TB) detection.
There were fleets of mobile units equipped for miniature films in most industrialized nations in the 1950s. TB is a public health concern because of its transmissibility. The screening was public-supported and involved a prodigious logistical effort wherever it was deployed.
It was abandoned in the 1970s, however, because the prevalence of TB had been forced down, skin testing had improved, and there was a greater focus on controlling ionizing radiation exposure. It was, though, the best generally applicable solution to an important public health concern at the time. Notably, chest x-ray screening for TB has been making a comeback in at-risk populations such as HIV-infected people and certain immigrant groups.
Screening mammography
Screening mammography seems to have followed a different trajectory, being a collection of separate local efforts continually fueled by an overwhelming barrage of popular media promotions. Like tainting a jury, this kind of public relations, though always well-intentioned, probably prevented an unbiased evaluation of the method itself.
In regard to studies supporting x-ray mammography, questions have been raised about the inherent validity of our most common tests for significance (i.e., Fisherian statistics) with many types of hypothesis-driven study designs. Dr. John Ioannidis made the case in a fascinating essay in 2005 that most published research findings are false.
The negative side has been met with several technical advances in x-ray mammography itself and a much deeper understanding of the molecular biology of breast cancer and the subdivision of "cancer" into subtypes with differing behaviors and drug sensitivities.
Dr. Nikola Biller-Andorno, PhD, and Dr. Peter Jüni shared their opinion that screening mammography should be discontinued in Switzerland in a recent issue of the New England Journal of Medicine (May 22, 2014, Vol. 370:21, pp. 1965-1967).
Noting that the first screening mammography trial was performed 50 years ago, Biller-Andorno and Jüni shared graphical representations of the real effect of mammography and how women perceive the effects, again emphasizing the potential bias of undocumented public thought in our interpretations of utilization studies or how we act on them.
Ultrasound and the breast
You must be wondering what, if anything, this has to do with ultrasound. It does relate -- perhaps more than you might think. One of the earliest papers on ultrasound was published by John Wild and John Reid in 1952 ("Further pilot echographic studies on the histologic structure of tumors of the living intact human breast"). This study used A-mode scanning to differentiate benign and malignant masses.
The notion of ultrasound screening was promoted in the 1980s by Dr. Elizabeth Kelly-Fry in Indianapolis and by my dear friend, the late Dr. Toshiji Kobayashi in Japan. For years I avoided this application, as I didn't think the equipment was ready.
One of the main problems ultrasound has always faced is that even a few clinical failures from technically premature use overshadow a lot of reliable uses in other applications. The other factor is that if you accept that diagnosis must be as early as possible, then you cannot beat x-ray detection of microcalcifications, which will precede the formation of a mass of abnormal cells.
I changed my mind, however, due to high-frequency, noise-suppressing equipment improvements in the past few years. Also, I had been finding a lot of advanced breast cancers with that equipment in referrals from alternate healthcare sources of patients who were fearful of MDs and who had not had any kind of surveillance other than some breast thermography, which I had thought had failed and disappeared in the 1970s.
Doing breast ultrasound is actually pretty easy. Cancers all arise in gland tissue that are the radially arranged reflective patches surrounding ducts. At higher frequencies and with noise suppression, cancers -- being more uniform in architecture -- are hypoechoic, improving inherent contrast against the parenchymal surround.
Automated breast ultrasound
Automated breast ultrasound systems (ABUS) are being promoted for screening. Seems reasonable, doesn't it? Actually, this may be the best ultrasound example I know of market-driven technology development in the absence of understanding of the clinical application.
Here's why: Detection of low-contrast targets is improved when they are moving. That has been known and studied forever. It's why that lonely sailor in the crow's nest scans the horizon instead of staring fixedly at it. Or, think of detecting a moving plane so far off that none of its features are evident. There is a sizeable collection of literature on contrast detection in static and moving images.
The automated systems have beautiful static displays, which seem to resemble the kind of images that mammographers, working with a static form of imaging, use daily. If you want to do breast nodule detection, forget automation, though, and have a human observer slide a transducer along radials from the nipple while watching the screen.
Most people who do breast ultrasound will tell you that is how you find lesions. When something has caught your eye, you go back and get static images of the nodule to document its location and features.
You could use your ABUS if you could page back and forth through the axis of the scan image set when you're reviewing the case, but that does not seem to be an efficient way for a clinic to utilize its personnel.
Ultrasonic breast architecture
The following unusual case will give us a good clue about another potential facet of ultrasound breast screening.
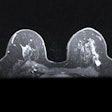
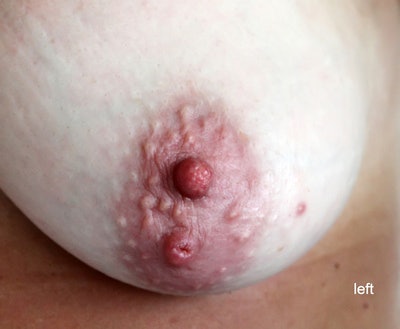 All images courtesy of Dr. Jason Birnholz.
All images courtesy of Dr. Jason Birnholz.This finding goes by the term "nipple dichotomy," and in this case it was unilateral. This is a duplication anomaly involving the entire breast, not a superficial cosmetic concern.
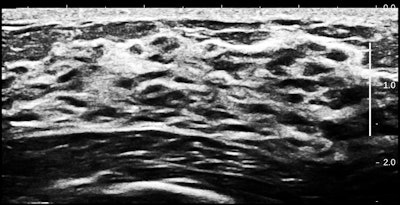
Inside the breast, there is twice the number of gland cells as in an ordinary breast. As you can see, parenchyma fills the space from the chest wall muscle to a relatively thin rim of fat. There is also duct ectasia.
If you assume that several sequential genetic events have to occur for a cancer to arise, it would seem reasonable that the more cells there are in a region, the greater the chance that one cell will turn bad. In breast terms, the more parenchyma there is, the greater the cancer risk.
Gland tissue is not distributed evenly in the breasts. Usually, most gland tissue is in the upper and outer quadrant, and that is where most tumors are found (as shown here in diagrams from the California Breast Cancer Research Program [CBCRP]).
Of course x-ray mammographers are well aware of this, with many papers relating mammographic density to breast cancer risk. Ultrasound acquires parenchyma distinctly from fat and is capable of exact geographic mapping. If you did such a study, on average the percentage total parenchymal volume would correspond to the CBCRP's cancer incidence distribution maps.
My own impression is that it is visually obvious when glandular volume is increased. However, it is possible to measure this ultrasonically, which will enable prospective evaluation. This can be done via segmentation by gray level and then counting pixels in the high range.
The volume of fat tends to determine overall breast size, but normal gland volume is reasonably constant from woman to woman. This is why breast cancer occurrence is not linked to breast size directly, and why ratios of gland to nongland components of the breast are not apt to provide worthwhile information.
Another anecdotal observation is that in women at high intrinsic risk for breast cancer, glandular atrophy with menopause is delayed and incomplete. Visually, there is way more parenchyma than there ought to be.
I suggest that we start thinking about ultrasound more seriously in a screening role. In the first instance, one may form a risk level based on glandular volume, separating low- and high-risk states. This will permit a rationally stratified approach to surveillance and test utilization.
Gland volume should be supplemented with genetic markers as they are developed and as those markers more truly represent risk for the whole population. Second, if a nodule is found, it is more likely to be significant than in situ lesions that have never progressed, and there will be typical ultrasound signs for its classification.
Dr. Jason Birnholz was one of the few advanced academic fellows of the James Picker Foundation, and he has been a professor of radiology and obstetrics. He is a fellow of the American College of Radiology and the Royal College of Radiology, and he was an associate fellow of the American College of Obstetricians and Gynecologists.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnie.com.