Women who have received the COVID-19 vaccine may benefit from scheduling their screening mammogram before vaccination or consider delaying screening by eight weeks, according to research published November 17 in Academic Radiology.
A team led by Dr. Sean Raj from the Baylor University Medical Center in Dallas also found that while the Pfizer-BioNTech vaccine may have an overall more robust immune response, the Moderna vaccine may have a stronger immune response in elderly women.
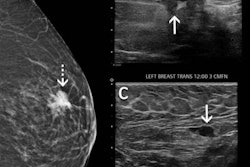
"In the context of a recent vaccination, these enlarged axillary lymph nodes are almost always part of the body's normal immune reaction," Raj told AuntMinnie.com. "However, enlarged axillary lymph nodes on mammography can also less likely represent a sign of cancer and lead to additional diagnostic testing."
While Raj led the research while at Baylor, he is now chief innovation officer at SimonMed Imaging.
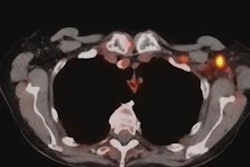
Since the first administration of the COVID-19 vaccine in December 2020, some women have presented with unilateral axillary lymphadenopathy on routine screening mammograms. While this may appear concerning in terms of breast cancer diagnosis, it can be a normal sign of an immune response to the vaccine.
Previous research suggests lymphadenopathy occurs most commonly between one and four weeks after vaccination. If it's due to an immune response from the vaccine, not malignancy, this means more cost for follow-up, as well as distress for patients.
"Further, it represents wasted healthcare resources in the form of primary care office visits, additional visits to referring physicians, and follow-up," the researchers added.
While research performed in the past year indicates axillary lymphadenopathy can be caused by the vaccine, it is not known what percentage of patients will develop subclinical unilateral axillary lymphadenopathy after receiving either the Pfizer or Moderna COVID-19 vaccine.
Raj et al wanted to determine the prevalence of such lymphadenopathy on screening mammography in women who received either the first or second dose of the vaccines compared with women who have not.
The team looked at data from 1,027 women between December 14, 2020 (when the vaccine was first made available), and April 14, 2021. Out of these, 43 were recalled for unilateral lymphadenopathy. Out of the 43 women, 34 received a COVID-19 vaccination ipsilateral to the lymphadenopathy (Pfizer, 19; Moderna, 15), while nine did not.
Unilateral axillary lymphadenopathy was more prevalent in women who received the vaccine within about seven weeks before mammography (p < 0.01).
The study authors found that 13.2% of patients who received the Pfizer vaccine and 9.5% of patients who received the Moderna vaccine developed lymphadenopathy. They also found that Moderna's vaccine had a more robust reaction in the elderly, with the average age of women presenting with lymphadenopathy who received the Moderna vaccine was 63.7 years compared to 59.7 years for the Pfizer vaccine and 56.4 years for the nonvaccinated group.
For both vaccines, lymphadenopathy naturally went away on an average of 46.5 days after the last COVID-19 vaccine, though this result did not achieve statistical significance.
The researchers said awareness of lymphadenopathy is "crucial" for radiology as a whole since reactive lymph nodes can also be visualized on imaging methods such as breast MRI, x-ray, or CT. They said awareness can improve scheduling screening mammograms around COVID-19 vaccinations, decreasing unnecessary healthcare costs for patients and healthcare systems.
However, women in high-risk groups for breast cancer will need special care and consideration when it comes to the question of delaying screening mammography, if even for a short time, Raj said.
The study authors also wrote that future research could include larger sample sizes and the Johnson & Johnson vaccine, which none of the patients in this most recent study received due to the time frame included.
Raj also said patients may have a similar reaction as COVID-19 booster immunizations roll out.
"The interesting question is will there be a similar incidence of subclinical axillary lymphadenopathy with the booster dose or potentially more or less due to an already 'primed' immune system? The results of this study may inform future policy or recommendations," he told AuntMinnie.com.