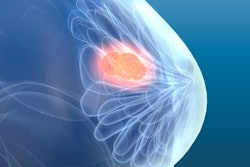
QT Imaging has received clearance from the U.S. Food and Drug Administration (FDA) to calculate fibroglandular volume (FGV) of the breast and the ratio of FGV to total breast volume on its QTscan breast imaging system.
FGV ratio can contribute to an assessment of risk for breast cancer, and changes in the ratio can be used to measure the efficacy of medication used to treat or prevent breast cancer, QT Imaging said.
 QT Imaging's QTscan system for breast imaging. Image courtesy of QT Imaging.
QT Imaging's QTscan system for breast imaging. Image courtesy of QT Imaging.The company's QT Scanner 2000 Model A is indicated for use as an ultrasonic imaging system to provide reflection-mode and transmission-mode images of a patient's breast. The system is not intended to be used as a replacement for screening mammography, the company noted.