Esophageal Carcinoma:
The prevalence of esophgeal cancer has increased dramatically over the past 30 years (350-800% increase in adenocarcinoma, although squamous cell carcinomas still account for about 85% of cases [7]) [1]. Early stage esophageal cancer is often asymptomatic and detection is uncommon [3]. As a consequence, esophageal cancer usually presents at an advanced stage with 75% of patients having lymph node metastases at presentation. Overall survival rate at 5-years is 10% or less, however, patients with early stage cancer can have survival rates between 57% to 78% [3,6,7].
The two most important prognostic indicators for esophageal carcinoma are depth of tumor penetration and nodal involvement [7]. Esophageal carcinoma is staged based upon a TNM classification system [7]. For patients with esophageal tumors remaining in the wall of the esophagus the 5-year survival is approximately 40% [7]. If the tumor invades the adventitia, survival drops to 4% at 5 years [7]. Patients with advanced, but potentially resectable tumors generally receive neoadjuvant chemotherapy, radiation therapy, or both followed by surgery which results in improved disease free survival (5 year survival is 20-30%) [6,8]. Patients with lymph node metastases have a 5 year survival of only 3%, compared to 40-42% without nodal involvement [1,7]. Distant metastases are found in about 18% of patients at presentation- especially to abdominal nodes, liver, and lung. Involvement of cervical or celiac lymph nodes is considered metastatic disease [6].
Treatment for esophageal carcinoma depends on accurate staging [6]. Surgery for operative candidates is not without significant risk and is associated with a mortality between 5 to 20% [1]. Moreover, the overall survival after "curative" resection does not exceed 25% which indicates micrometastases or undetected metastases were likely present at the time of surgery [10]. Proper identification of patients that are truly surgical candidates is crucial so that patients with advanced stage disease can be referred for prompt multimodality treatment protocols [3].
Although endoscopic evaluation and CT imaging form the mainstays of clinical staging, they cannot accurately identify all sites of disease [3]. FDG PET imaging holds great promise as a means to fully assess for the presence of unsuspected sites of disease in patients presenting with esophageal carcinoma. PET in particularly useful in reducing the number of unnecessary surgical procedures through the identification of unsuspected sites of disease [10]. Up to 20% of patients can be upstaged due to the presence of metastatic disease identified on PET imaging [10].
FDG PET imaging is presently reimbursed for the following indications:
1- Pre-surgical staging and restaging of esophageal carcinoma
2- Monitoring for recurrence (may be covered unless strong negative evidence is present)
Primary tumor:
Despite reported sensitivities between 91-100% for demonstrating primary esophageal cancer (both squamous cell and adenocarcinoma), FDG PET imaging lacks sufficient spatial resolution and boundary determination to accurately assess T-stage [9,10]. False negative exams can be seen in cases of carcinoma-in-situ and T1 (small) cancers [6,10]. False positive exams can occur due to esophageal inflammation and there can be normal mild FDG activity in the esophagus possibly due to swallowed saliva or smooth muscle metabolism which could potentially obscure subtle lesions [1]. PET imaging is also not sensitivity for assessing for the presence of local invasion [1].
Nodal metastases:
Metastases to lymph nodes is one of the most important prognostic factors in patients with esophageal cancer [6]. Both the number and the location of involved lymph nodes affect prognosis [6]. FDG PET is more sensitive than CT for depicting nodal metastases in patients with esophageal cancer [6]. CT has a sensitivity of 31%-44% for detecting N1 nodes and 14%-29% for detecting M1a nodes (cervical or upper abdominal nodes), compared to 55%-64% and 43%-71%, respectively, for FDG PET imaging [6,10]. CT has an accuracy of 40-73% for the detection of pathologic mediastinal nodes (using a 1 cm size criteria) [1,6]. The reported accuracy of FDG PET in the staging of locoregional lymph node metastases varies from 24-90% [1,6].
Some of this variability in the accuracy of PET imaging is related to the limited spatial resolution of PET imaging and local nodal metastases adjacent to the primary tumor may be obscured by tumor activity [1,9,10]. Additionally, para-esophageal nodes are usually small in size and may contain only microscopic foci of tumor [9]. These nodes are generally best depicted by trans-esophageal ultrasound [10]. Although the presence of local para-esophageal nodal metastases carries prognostic information, the presence of these metastases does not preclude curative surgical resection (for example, they do not necessarily change clinical management) [1]. However, supraclavicular, cervical, and celiac lymph node metastases are considered M1a disease [1]. The presence of pathologic adenopathy in these other sites would substantially alter patient management and these sites of disease are more accurately detected with PET imaging [6,10].
|
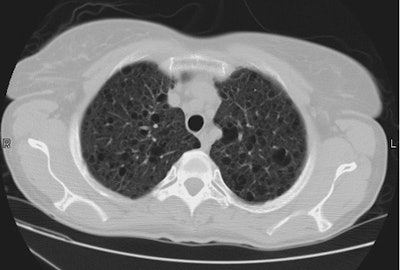
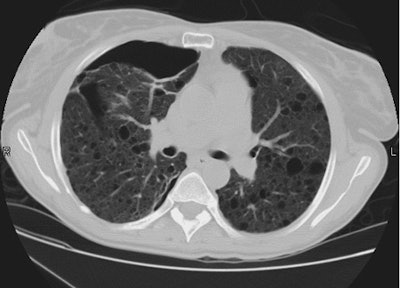
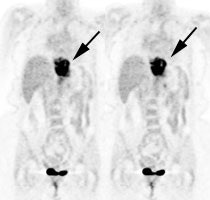
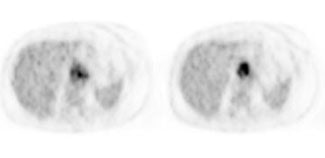
Esophageal carcinoma: The patient shown below presented with a history of progressive dysphagia. A barium swallow revealed a distal esophageal mass. CT and FDG PET imaging were performed for patient staging. The large esophageal mass (white arrows on CT) demonstrated intense tracer uptake (black arrows on PET scan). Uptake could also be seen in regional gastrohepatic ligament nodal metastases (right images). Retrocrural metastases seen on CT blended imperceptibly with the primary tumor on PET imaging. The was no evidence of distant metastatic disease. (Click here to view rotating volume image [1.5 MB])
|
|
|
Distant Metastases:
The presence of distant organ metastases (M1b disease) in esophageal cancer has considerable impact on patient management, as patients are then considered non-resectable. Unfortunately, over 30% of distant metastases are radiographically occult [10]. The major advantage of FDG PET over conventional imaging is the ability to detect distant metastases [1,10]. In a prospective study of 100 patients with esophageal carcinoma referred for surgery, FDG PET imaging was shown to be more accurate than CT in detecting distant metastases (84% versus 63%) [4]. The likelihood for the presence of distant metastases increases with the T-stage of the tumor- particularly with T3 lesions [10].
FDG-PET has a reported sensitivity of 69%-100%, a specificity of 84%-90%, and an accuracy of 84%-91% for the evaluation of distant metastases [1, 3, 4,10]. Sensitivity of CT for distant metastases has been reported to be as low as 30%-46% [4,10].
FDG-PET can demonstrate metastatic disease not identified on conventional studies in about 9% to 28% of patients [1,3,4,5]. PET exams can also exclude metastatic disease at sites considered abnormal on conventional imaging. FDG PET exam results can affect patient management in approximately 22% of patients [5]. Metastatic lesions not identified on FDG-PET exams are typically less than 1 cm in size and are usually in the lung or liver [4].
PET imaging may also provide prognostic information. When PET exams demonstrate only local disease, the 30-month survival has been reported to be up to 60%; while patients with distant disease on PET exams had a 30-month survival of 20% [4]. Other findings on the PET exam which are associated with a worse prognosis include increasing maximum SUV of the primary tumor, tumor length on the PET scan (over 5 cm), number of PET-positive nodes, and increasing PET stage [11].
|
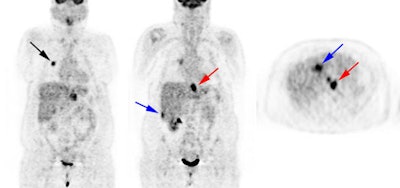
Metastatic esophageal carcinoma: The patient presented for evaluation of a pulmonary nodule. PET revealed tracer uptake in the nodule (black arrow), as well as in an esophageal mass (red arrow), para-esophageal adenopathy, and liver metastases (blue arrows). |
|
|
|
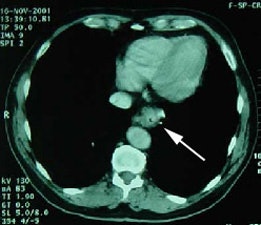
Unsuspected metastatic esophageal carcinoma: The patient shown in the case below was referred for FDG PET imaging for the evaluation of esophageal carcinoma. A CT scan demonstrated abnormal thickening of the distal esophagus (white arrow), but no evidence of metastatic disease. The PET exam revealed long segment abnormal increased tracer activity within the primary esophageal lesion, as well as an unsuspected bone metastasis to the right hip (black arrow). Such findings on PET imaging have tremendous impact on patient management. Case courtesy of CTI.
|
|
|
Response to therapy:
FDG PET imaging can also be used to evaluate response to neoadjuvant therapy [5]. A decrease in FDG uptake has been found to be significantly greater in patients that are responding to therapy [5]. The sensitivity and specificity of serial FDG PET studies for determining therapy response are 71% and 82%, respectively [8]. In patients that are receiving radiation therapy, a 2 to 3 month delay is recommended prior to PET imaging to avoid false-positive exams associated with radiation esophagitis [5]. Surgery performed within 4 weeks of the FDG exam can result in false-positive findings- it is recommended that scans be delayed for at least 6 weeks following surgery [8].
|
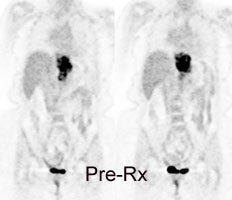
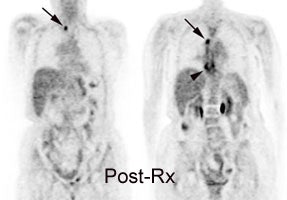
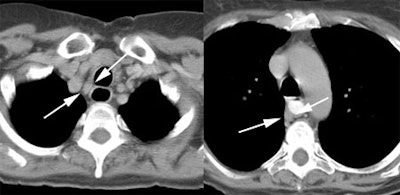
Response to therapy: This is the patient shown in the case above with progressive dysphagia and a large distal esophageal mass with gastrohepatic ligament adenopathy. Note the decreased uptake within the primary mass (arrowhead) and gastrohepatic nodes following initiation of radiation therapy. However, new uptake can be seen in two lymph nodes (black arrows) which were not identified prospectively on post-therapy CT imaging (white arrows on CT). The findings are concerning for metastatic disease and the patient went on to receive additional chemotherapy with subsequent resolution of the nodal uptake. |
|
|
Recurrent Esophageal Cancer:
Even after apparently curative surgery, the overall 5 year survival for esophageal carcinoma is only 30-50% [2]. Two-thirds of patients have a recurrence within one year and nearly all within 2 years after primary operation [2]. In about one-third to half of patients, the recurrence is located in the operative field (to regional lymph nodes or a recurrent mass) [2,6]. The remainder, and majority of recurrences are distant metastases [2]. Some reports suggest that early detection and aggressive treatment of recurrent disease may result in prolonged tumor-free survival and occasionally cure [2]. A multimodality approach is currently used for the detection of recurrent esophageal carcinoma [9]. FDG PET imaging allows a highly sensitive means for whole-body staging in patients with suspected recurrent esophageal carcinoma [2].
For the diagnosis
of perianastomotic recurrence FDG-PET compares well with conventional imaging.
In one study PET imaging had a sensitivity of 100%, specificity of 57%, and an
accuracy of 74% (compared to 100%, 93%, and 96% for conventional imaging) [2].
However, conventional imaging studies cannot reliably differentiate post
surgical change/scar from tumor recurrence. FDG-PET is extremely useful in this
setting [1]. By demonstrating increased metabolic activity within an abnormality
detected by conventional imaging, a greater confidence in the diagnosis of
recurrent disease can be achieved. Additionally, PET imaging can help to guide
diagnostic biopsy to the regions of greatest metabolic activity. False-positive
exams can be seen in association with recent endoscopic stricture dilatation (up
to 2 months post procedure) [2].
|
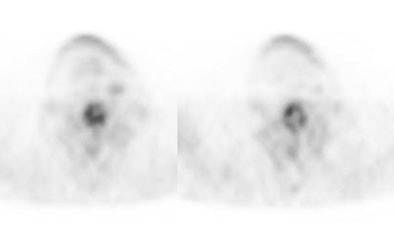
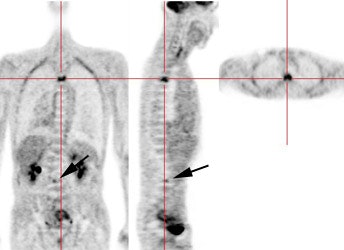
Recurrent esophageal cancer: The patient below had a history of esophageal cancer that had been treated with esophageal resection and gastric pull through procedure. The patient had a new pleural effusion and PET scan was performed as part of the evaluation for recurrent esophageal carcinoma. The PET scan revealed unsuspected bone metastases to the thoracic (cross-hairs) and lumbar spine (black arrow). |
|
|
REFERENCES:
(1) Radiographics 2000; Skehan S, et al. Imaging features of primary and recurrent esophageal cancer at FDG PET. 20: 713-723
(2) J Thorac Cardiovasc Surg 2000; Flamen P, et al. The utility of positron emission tomography for the diagnosis and staging of recurrent esophageal cancer. 120: 1085-92
(3) Thorax 1998; Lowe VJ, Naunheim KS. Current role of positron emission tomography in thoracic oncology. 53: 703-712
(4) Ann Thorac Surg 1999; Luketich JD, et al. Evaluation of distant metastases in esophgeal cancer: 100 consecutive positron emission tomography scans. 68: 1133-37
(5) Radiographics 2003; Kostakoglu L, et al. Clinical role of FDG PET in evaluation of cancer patients. 23: 315-340
(6) Radiology 2003; Yoon YC, et al. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection- prospective study. 227: 764-770
(7) AJR 2003; Iyer RB, et al. Diagnosis, staging, and follow-up of esophageal cancer. 181: 785-793 (No abstract available)
(8) J Nucl Med 2004; Kostakoglu L, Goldsmith SJ. PET in the assessment of therapy response in patients with carcinoma of the head and neck and of the esophagus. 45: 56-68
(9) Radiology 2004; Rohren EM, et al. Clinical applications of PET in oncology. 231: 305-332
(10) J Nucl Med 2004; Heeren PAM, et al. Detection of distant metastases in esophageal cancer with 18F-FDG PET. 45: 980-987
(11) J Nucl Med 2004; Choi JY, et al. 18F-FDG PET in patients with esophageal squamous cell carcinoma undergoing curative surgery: prognostic implications. 45: 1843-1850