PET CNS Imaging:
Seizure Disorders:
(For more extensive discussion, see Tc-HMPAO seizure imaging in CNS section)
Epilepsy is one of the most prevalent neurological
disorders. Seizures can be classified as either partial (focal) or generalized.
Partial seizures originate in a given area of the brain and can be divided into
simple (with no impairment of consciousness) and complex (with impairment of
consciousness). Between 10% to 20% of patients with partial complex seizures
have inadequate control on medical treatment. Patients unresponsive to anti-convulsant
therapy may be candidates for surgical treatment. Most partial complex seizures
originate in the temporal lobe, unfortunately scalp EEG often fails to
accurately localize the seizure focus. Depth EEG is much more accurate, but it
is also extremely invasive (requiring a craniotomy) and suffers from regional
under sampling.
PET is very sensitive in the early detection of the functional disturbance associated with microscopic neuronal disorganization which results in epilepsy. During the interictal phase, PET imaging with FDG will demonstrate a zone of decreased glucose utilization and decreased cerebral blood flow (CBF) at the site of a seizure focus in 60 to 70% of patients with normal MRI's [5]. The distribution of the PET abnormality correlates very well with the extent of the epileptogenic zone as determined by intraoperative EEG, but correlates poorly with the extent of morphological abnormalities in the resected specimen- the area of hypometabolism is often much larger than the actual area of structural abnormality. Among patients undergoing temporal lobectomy, a quantitative asymmetry index of greater than 15% for the lateral temporal lobe indicates the patient will likely have a good surgical outcome [6].
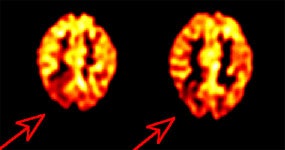
Inter-ictal FDG PET exam: The patient in the case below suffered from intractable drop seizures since the age of 18 months. An MRI was normal. Ictal scalp EEG demonstrated epileptiform activity emanating from the right parietal region. The FDG PET exam revealed a region of hypometabolism (red arrow) in the right parietal lobe. The PET exam guided subdural grid placement with very good correlation. The patient underwent surgical treatment and became seizure free. The exam was performed on an ECAT EXACT HR+ (manufactured by CTI). Case courtesy of Harry Chugani, M.D., Childrens Hospital of Michigan, and CTI PET Systems, Inc. |
|
In patients with Sturge-Weber syndrome, FDG typically shows widespread unilateral hypometabolism ipsilateral to the facial nevus [11]. In infants less than 1 year of age, however, the interictal scan often shows a paradoxical increased cortical metabolism ipsilateral to the facial nevus [11].
Cerebrovascular Disorders
Physiology, radiopharmaceuticals, and the PET exam:
Cerebral blood volume, cerebralblood flow, the cerebral metabolic
rate of oxygen, and cerebral metabolic rate of glucose all reflect various
aspects of cerebral homeostasis. Derangements in cerebral perfusion produce
alterations in this homeostasis that can be images with PET agents.
Cerebral blood volume:
With PET imaging, the regional distribution of cerebral blood volume (CBV) is
imaged following the inhalation of C-11 (T1/2= 20.3 min.) or O-15 carbon
monoxide (CO). Radiolabeled CO binds with high affinity to hemoglobin within red
blood cells (forming carboxyhemaglobin) permitting absolute measurements of CBV.
Images of CBV show the greatest concentration of activity in the dural venous
and carotid sinuses. Although blood accounts for only 5% of the volume of the
gray matter structures, the gray matter has a larger CBV than the white matter. Thenormal whole brain CBV value is 4.2 ml/100gm.
Vasodilatation is an earlyresponse to cerebral arterial occlusion and can be used to identify areas ofdecreased perfusion pressure. The ratio of CBV/CBF (cerebral blood flow)increases as cerebral perfusion pressure falls.
Cerebral blood flow:
Cerebral blood flow (CBF) can be measured by using O-15 water intravenously (for
scanners with high count rate capabilities), O-15 carbon dioxide inhaled (for
scanners with limited count rate capabilities), or C-11 butanol. O-15 labeled
carbon dioxide is converted to radiolabeled water by the action of carbonic
anhydrase. The whole brain mean CBF isbetween 40-50 ml/100 gm/min. The gray matter CBF is 2.5 to 3 times
greater than that of the white matter (56 versus 20 ml/100gm/min.). Electrical
activity in the brain diminishes at CBF values below 20 ml/100gm/min. and cell
death occurs when the CBF falls below 10 ml/100gm/min.
Cerebral metabolic rate ofoxygen: The cerebral metabolic rate ofoxygen (CMRO2) is measured using O-15 oxygen. Anormal mean regional CMRO2is approximately 3.0 ml O2/100gm/min.A minimum value of at least 1.3-1.5 ml O2/100gm/min. is required for
tissue viability. CMRO2 is calculated from measurements of CBF, CBV,
and the oxygen extraction fraction (OEF).
The OEF represents the fractionof oxygen extracted from the blood by the brain in a single capillary transitand is approximately equal to 0.4. OEF increases as blood flow decreases. OEF iscalculated by using O-15 labeled O2 (O15O). Labeled O2 is
extracted by the brain and immediately metabolized to H2 15O.
OEF=CMRO2 / CBF [arterial O2concentration]
Cerebral metabolic rate for
glucose: Either F-18 fluorodeoxyglucose or C-11 deoxyglucose can be used to
determine cerebral metabolic rate for glucose (CMRglu). Unfortunately, C-11
deoxyglucose is a less than optimal agent, because it is metabolized and the
C-11 rapidly leaves the brain in the form of labeled CO2. FDG crosses
the blood brain barrier by a carrier mediated transport mechanism similar to
those operating for glucose. The agent is phosphorylated into hexokinase, but
not further metabolized and it remains inside the neural cell.
Images are performedapproximately 40-60 minutes after tracer injection at which time most blood-poolactivity has cleared. The mean CMRglu is
approximately 5 mg of glucose per 100 gm/min. (or 30 umol/min./100gm).
The gray-to-white matter utilization ratio is approximately 3-to-1 and
homogeneous regional utilization of glucose is seen within the gray matter
structures of normal resting individuals (although a decline in frontal
metabolism has been reported with age).
Cerebral Infarction
In a normal resting brain,cerebral blood flow, glucose metabolism, and the cerebral metabolic rate for O2
are linked by a constant relationship and the OEF is relatively homogeneous
across the brain. With a reduction in cerebral perfusion pressure, arteriolar
vessels dilate in an effort to maintain regional cerebral blood flow (rCBF). CBV
will be increased in these regions [10].
When the vascular response ismaximal and no further dilatation is possible, rCBF will begin to decrease,however, regional oxygen extraction fraction (rOEF) will begin to increase in aneffort to maintain a stable CMRO2 [10]. With mild to moderate
decreases in CBF, neuronal function can be maintained without compromise so long
as OEF increases to maintain CMRO2. In severe ischemia (as CBF falls
below 15-18 ml/100gm/min.), oxygen extraction rises to maximal levels. Further
decreases in perfusion will then lead to a decrease in CMRO2. The
presence of increased OEF has been shown to be a powerful and independent
predictor of subsequent stroke in patients with atherosclerotic cerebral
vascular disease [7].
Regions with CBF below 11ml/100gm/min. almost always proceed to tissue necrosis. Focal reductions in CBFand CMRO2 mimicking acute infarction can be seen in patients with
other disorders such as cerebral hemorrhage, multiple sclerosis, or infiltrating
tumor. In general, CMRglu measured with FDG tends to be lower in a site of
hemorrhage compared to acute ischemic infarction, although there is considerable
overlap.
In most cases of acute ischemic stroke, the CMRglu is reduced in the affected tissue; however, CMRglu may be increased above normal gray matter in some sections of the infarction in up to 10% of cases probably secondary to increased anaerobic glycolysis. In general, CMRglu is reduced less than CMRO2. During acute ischemia the CMRO2 appears to be the most important determinant of tissue viability. During the early portion of the post-infarction period, depending on the site and severity of obstruction, ischemic cerebral tissue may be salvageable. Tissue pH is reduced under conditions of acute ischemia, but is typically alkalotic by 5 days following the infarction.
By one week post infarction there is a reversal of the previous pattern noted, with increased rCBF associated with decreased or unchanged glucose utilization [10]. This uncoupling of flow and metabolism in which the CBF exceeds metabolic demands represents luxury perfusion. By one month post-infarct, a matched decrease in rCBF, rCMRO2, and glucose metabolism is seen indicating irreversibly infarcted tissue. Arteriovenous malformations (AVMs) also create a situation in which local blood flow greatly exceedslocal oxygen utilization and cannot be distinguished from a subacute infarctionwith luxury perfusion by PET imaging.
| Condition | CMRO2 | CBV | OEF | CBF | CMRglu |
| Decreased flow reserve | Normal | Increased | Increased | Maximum possible | Normal |
| Acute ischemia | Decreased | Increased | Increased | Decreased | Generally reduced |
| Late infarct | Decreased | Variable | Normal | Decreased | Decreased |
PET imaging has also demonstrated widespread metabolic changes in structures remote form the site of infarction [10]. Crossed cerebellar diaschisis refers to a decrease in cerebellar blood flow and oxygen metabolism contralateral to the site of cerebral infarction during the first 2 months post event [10]. Interruption of the cerebro-ponto-cerebellar pathway is the proposed mechanism for this finding. Other remote function effects include decreased metabolism in the ipsilateral thalamus and caudate [10]. A decline in metabolic activity in the primary visual cortex ipsilateral to an infarct which involves the anterior visual pathways reflects a clinical homonymous hemianopsia.
Multi-infarct Dementia
MID is characterized by multifocal, asymmetric matched reductions of rCBF and rCMRglu corresponding to the locations of cortical and subcortical infarcts [8].
Arteriovenous Malformations
These lesions classically demonstrate increased regional cerebral blood volume ipsilateral to the malformation, associated with significantly decreased glucose metabolism.
Neuropsychiatric Disorders:
Dementia:
PET imaging has been used for the assessment of dementia. When performing quantitative measurements on PET exams performed on elderly patients for dementia, it is important to remember to correct for age-related volume loss. Partial-volume averaging due to expanded sulci can result in PET measurements that underestimate true values [1]. When such corrections are made, it can be demonstrated that there is no decline in cerebral blood flow in healthy elderly individuals (except for the oribitofrontal cortex) [1,2]. A decline in dopamine activity with age, however, has been documented [2].
Clinical:
Alzheimer disease (AD) is a progressive neurodegenerative disorder associated with gradual deterioration in cognition, function, and behavior [16]. Decreasing mortality with an ever greater elderly population has led to a rising prevalence of senile dementia [4]. It is estimated that 8% of people over the age of 65 years suffer from Alzheimer's disease and the prevalence climbs with increasing age (about 30% in patients over the age of 85 years) [15,16]. Alzheimer's disease (AD) affects over 4 million people in the United States and the condition is tremendously costly to patients, their families, and society [4]. AD progresses incidiously with initial sparing of sensory and motor function [15,16]. Unfortunately, the condition frequently goes unrecognized or is not properly clinically diagnosed until it's more advanced stages [15,16]. Recent studies have identified certain genetic risk factors for Alzheimer's disease. Apolopoprotein (APOE), a gene on chromosome 19, has been reported to have an association with AD. The presence of APOE-4 allele increases the risk for AD, while the APOE-2 allele appears to have a protective effect. Patients with the APOE-4 allele may prove to benefit from early PET imaging in order to identify changes suggestive of AD prior to the onset of clinical symptoms.
A need for accurate, early diagnosis is very important as medications (cholinesterase inhibitors) are now available that have been shown to improve or delay the memory and other cognitive loss that occurs in mild to moderate disease [15,16,20]. A delay in the implementation of therapy may also have long term consequences [20].
Pathology:
Pathologically AD is characterized by a reduction in the number of large cortical neurons in the temporal and frontal cortex and by the appearance of senile (amyloid) plaques and neurofibrillary tangles [16]. Cerebral metabolic rate for glucose primarily reflects neuronal and synaptic activity, and at autopsy patients with Alzheimer's are found to have an average decrease of 50% in the density of the granular neurophil in the parietal and temporal cortices [8]. The greatest density of neuritic plaques and neurofibrillary tangles are also found in these locations [8].
PET imaging in Alzheimer's disease:
FDG PET can improve diagnostic accuracy in the evaluation of patients with cognitive decline. PET FDG imaging is currently used primarily as an adjunct to the clinical determination of AD and is especially useful in differentiating AD from other dementias (including vascular dementia). [16,17]. FDG PET imaging has demonstrated promise in providing a diagnosis of AD 2 to 3 years before full dementia-related symptoms manifest [16]. By confirming the diagnosis of AD, PET imaging allows for the early institution of appropriate therapy. Also- patients with negative PET scans, can be spared the expense of unnecessary treatment. Presently, the available literature would also support the use of PET imaging for at least 2 groups of patients- those with mild or moderate cognitive impairment who meet the standard criteria for dementia (the cause of which has not been identified by an appropriate medical workup); and those patients with mild or moderate cognitive impairment who exhibit progressive cognitive dysfunction that has not reversed after a thorough medical workup [20].
Findings in AD: AD classically produces a pattern of bilateral parietotemporal hypometabolism [3] (which can be asymmetric early in the course of the disease [16,20]) even before substantial abnormalities appear on clinical or neuropsychiatric tests [8]. There is relative sparing of the basal ganglia, thalamus, cerebellum, and cortex mediating primary motor and sensory functions [20]. The combination of reduced metabolic activity in a suspicious distribution and genetic risk factors provides a means for the detection of pre-clinical disease [18].
PET FDG imaging has been shown to have a sensitivity of 92-96%, specificity of 63-71%, and an accuracy of 82-87% for the confirmation of Alzheimer's in patients that are difficult to characterize on clinical evaluation [3,4]. In another study of 138 patients with a pathologically confirmed diagnosis, PET correctly identified the presence or absence of AD in 88%, with a sensitivity of 94% (89-99%; 95% CI), and a specificity of 73% (60-87%, 95% CI) [17]. The patients in this study were generally felt to have mild cognitive impairment at the time of their initial PET exam [17]. PET imaging can also improve the accuracy in identifying early Alzheimer's without adding to the overall costs of diagnosis and treatment [15]. In patients presenting with cognitive symptoms, a negative PET indicates a lower likelihood for a progressive dementia with a specificity of about 76% [17]. In a meta-analysis of PET data in Alzheimer's disease, the summary sensitivity was 86% (95% CI: 76%-93%) and the summary specificity was 86% (95% CI: 72%-93%) [19].
When compared to SPECT imaging, reductions in tracer activity are significantly more pronounced for PET imaging [14]. The magnitude of the deficits on PET also correlates with the degree of cognitive impairment [16] and the correlation between dementia severity and the number of abnormal voxels is closer for PET than for SPECT [14]. Metabolic activity in the primary sensory-motor cortex, basal ganglia, thalamus, and cerebellum are typically normal in patients with Azlheimer's disease [16]. Overall, for the detection of AD, PET has an increased accuracy of 15-20% compared to SPECT imaging [20].
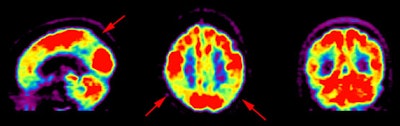
Alzheimer's disease on FDG PET: The case below is from an 86 year old male being imaged to exclude Alzheimer's disease. The FDG PET exam demonstrated bilateral temporoparietal hypometabolism. This finding is very sensitive for the diagnosis of Alheimer's disease. The exam was performed on an ECAT EXACT HR+ PET scanner (manufactured by CTI) after administration of 108 MBq FDG. The exam was acquired using a 3D dynamic emission and 15 minute transmission protocol. Case courtesy of Institut fur Medizin Forschungszentrum Julich, Germany and CTI PET Systems, Inc. |
|
The pattern of biparietotemporal hypometabolism is not absolutely pathognomonic for Alzheimer's and can also be seen in patients with Parkinson's disease with dementia [20], bilateral parietal subdurals [8], dementia with Lewy bodies (but will also involve the visual cortex) [16], Creutzfeldt-Jacob disease [3], or biparietotemporal infarctions [8].
The global CMRglu has also be evaluated in patients with AD and is reduced in up to 35% of Alzheimer's patients. However, this may also be seen in patients with depression, Parkinson's, following convulsion, trauma, cerebral irradiation, ECT therapy, and with drug induced impairment of consciousness. CMRglu also decreases normally in the elderly, despite maintenance of CBF, and CMRO2.
Abnormalities within the cholinergic system have been consistently identified in patients with Alzheimer's. There is a 40 to 90% decrease in the enzyme choline acetyltransferase and consequently in acetylcholine levels within the cerebral cortex. In contrast, the dopaminergic system is not abnormal in Alzheimer's patients (as it is in patients with Parkinsons).
New radiotracers may hold promise for imaging patients with AD [16]. The agent [18F] FDDNP targets amyloid senile plaques and neurofibrillary tangles and will show increased temporal-parietal accumulation of the tracer in AD [16]. The greater degree of tracer accumulation correlates with memory performance scores and decreased glucose metabolism on FDG PET imaging [18].
Picks disease is much less common than Alzheimers and it is also more difficult to diagnose clinically [8]. Pick's usually presents with changes in personality and behavior, with memory dysfunction occurring later [8]. At autopsy, there is frontal and temporal atrophy with neuronal loss, gliosis, inflated neurons, and Pick bodies [8]. Hypometabolism in the frontal and anterior temporal lobes has been seen in the few patients studied.
Parkinson's is a neurodegenerative disorder resulting from the progressive death of dopaminergic neurons in the nigrostriatal pathway (pars compacta of the substantia nigra and locus ceruleus, and presynaptic dopaminergic nerve terminals in the caudate nucleus and putamen) [8,12]. Symptoms consist of rigidity, bradykinesia, difficulty in initiating and stopping movement, and a resting tremor [8].
Motor disturbances begin only after a loss of approximately 70-80% of striatal dopamine- thus, there is a long latent period which precedes the development of clinical symptoms [12]. Approximately 10% of patients with Parkinson's develop a dementia [8] and biparietal hypometabolism identical to Alzheimer's is seen in these patients (this finding is typically not found in Parkinson's patients without dementia) [8].
The loss of dopaminergic nerve terminals in the basal ganglia can be detected by PET imaging with 6-[18F] fluoro-DOPA (an analog of the dopamine precursor dihydroxyphenylalanine [DOPA]) to study the presynaptic component of the nigrostriatal dopamine system and dopamine metabolism. 6-FD is largely metabolized peripherally by DOPA decarboxylase (DDC) to F-18 fluorodopamine before penetrating the BBB. This factor, as well as other peripheral metabolites which may cross the BBB, complicate the brain uptake of 6-FD. Inhibitors of DDC, such as Carbidopa, when injected before administration of the 6-FD improve brain uptake of the tracer considerably [Seminars, Oct. 94, p.339].
The cerebral radioactivity measured after the administration of 6-FD is a function of uptake of the isotope into the brain and its subsequent metabolism to dopamine. In Parkinson's disease, the decrease in 6-FD accumulation is much more severe in the putamen than in the caudate nucleus [Seminars, Oct. 94, p.339].
F-18 labeled derivatives of m-tyrosine have also been used to study Parkinson's disease. m-Tyrosine is a substrate for the enzyme aromatic amino acid decarboxylase which is responsible for the conversion of DOPA to dopamine. Marked reduction in activity within the basal ganglia (putamen and caudate) is seen in patients with Parkinson's disease with both 6-FD and m-tyrosine.
PET studies of alterations in D2 dopamine receptors have been conflicting. Patients that have been studied with C-11 N-methylspiperone while maintained on their usual dose of L-DOPA display essentially normal levels of dopamine receptors, despite the fact that F-18 fluoro-DOPA uptake is markedly reduced.
C-11 Nomifensine has been developed to study the dopamine reuptake system located on the presynaptic nerve terminals. This agent also demonstrates decreased uptake within the basal ganglia of Parkinson's patients.
Huntington's disease is a slowly progressive autosomal dominant neurodegenerative disorder characterized by choreiform (involuntary) movements, personality disorders, psychiatric symptoms, and dementia [8]. The age of onset is variable, but it occurs most frequently in the 3rd and 4th decades of life [8]. There is severe neuronal loss and atrophy of the caudate nuclei, and to a lesser extent the putamen and globus pallidus [8]. The caudate and putamen are deficient in the inhibitory neurotransmitter gamma aminobutyric acid (GABA) and glutamic acid decarboxylase.
FDG PET studies demonstrate decreased metabolic activity in the caudate, even when the CT is normal and years before the onset of motor symptoms [8,13]. Unfortunately, there is overlap of local caudate metabolic rates in patients at risk for Huntington's and normal control populations [13]. Global CMRglu tends to be normal in Huntington's patients, in contrast to Alzheimer's patients in which it is decreased.
Normal Pressure Hydrocephalus:
Clinically patients present with dementia, a gait disturbance, and urinary incontinence. ("Wet, wacky, and wobbly".) CT demonstrates moderate to severe enlargement of the frontal horns, obliteration of the sulci over the convexities, and periventricular hypodensities. The CSF pressure is normal. PET studies have demonstrated decreased metabolic activity compared to normal controls, but no regional deficit has been identified.
Dementia with Lewy Bodies:
Patients present with detailed visual hallucinations, Parkinson-like symptoms, or alterations in alertness and attention [16]. Cholinesterase inhibitors are currently the treatment of choice [16]. On FDG PET imaging there are bilateral temporal-parietal deficits similar to Alzheimers (AD), but the deficit also involves the occipital lobes (visual cortex) and cerebellum (areas typically spared in AD) [16].
Progressive supranuclear palsy:
PSP begins in the 6th or 7th decade of life, and the duration of illness can range from 2 to 12 years [8]. Clinical signs include rigidity (which is more prominent axilly than in the extremities), bradykinesia, supranuclear gaze palsies, pseudobulbar palsy, and dementia [8]. A clinical hallmark of the disorder is paralysis of vertical gaze, particularly in the downward direction [8].
Gross anatomic changes include atrophy of the midbrain, pons, dentate nuclei of the cerebellum, and the cerebellar hemispheres to some degree [8]. There is an increase in aqueductal size, as well as in the size of the quadrigeminal, ambient, and crural cisterns [8]. Classically the condition produces a pattern of global reductions in cerebral oxygen and glucose metabolism that are most prominent frontal cortex [3,8].
PET imaging findings in Dementias [20]
| Dementia | PET findings |
| Alzheimer's | Decreased activity in the bilateral temporal and parietal cortices. During early stages, the defects often appear asymmetric. There is relative sparing of the basal ganglia, thalamus, cerebellum, and cortex mediating primary motor and sensory functions. |
| Vascular | Scattered defects in the cortical, subcortical, and cerebellar areas |
| Frontotemporal (Pick's) | Frontal cortex and anterior temporal and mesiotemporal decreased activity with sparing of the sensorimotor and visual cortices |
| Huntington's | Decreased activity in the caudate and lentiform nuclei (early) with gradual development of diffuse cortical involvement |
| Parkinson's with dementia | Deficits are similar to those of AD, but with more sparing of the mesiotemporal area and less sparing of the visual cortex |
| Dementia with Lewy bodies | Deficits are similar to AD, but with less sparing of the occipital cortex and possibly cerebellum |
Psychiatric disorders:
Schizophrenia
Schizophrenia represents a heterogeneous disorder [9]. The most frequently reported finding in patients with schizophrenia is decreased metabolic activity and decreased CBF in the frontal cortex- referred to as hypofrontality [9]. This finding is predominantly seen in patients with chronic schizophrenia that have a long history of neuroleptic treatment [9]. Studies have shown that chronic neuroleptic treatment increases whole brain metabolism, but this increase is less in the frontal lobes than other areas [9]. In contrast, younger patients may not demonstrate hypofrontality [9]. Hypofrontality is also more commonly identified in paranoid (45%), than non-paranoid schizophrenics (20%).
When challenged with an activation task such as the Wisconsin Card Sorting Test, most patients with schizophrenia demonstrate a failure of frontal lobe activation which is felt to be related to decreased dopamine function [9]. Because the frontal cortex is highly interconnected, an abnormality in this location may produce disturbances of activity in other cortical and subcortical structures. Unfortunately, frontal metabolic defects are not specific for schizophrenia and have also been identified in patients with affective disorders, chronic cocaine abuse, Parkinson's disease, supranuclear palsy, and advanced age [9].
Several tracers have also been used to measure dopamine receptors in schizophrenic patients and patients with Parkinson's disease: 18-F or 11-C N-methylspiroperidol (NMS), 11-C raclopride, F-18 spiperone, and Bromine-76 bromospirone. N-methylspiroperidol (NMS) is a potent antipsychotic drug that is pharmacologically similar to haloperidol. It binds to both dopamine D2 and serotonin S2 receptors (however, its binding affinity for Serotonin receptors is much lower than for the D2 receptors). D2 receptors exist on axons passing from the substantia nigra to the basal ganglia, while D1 receptors are found on axons passing from the cerebral cortex to these structures.
Following administration of NMS there is preferential accumulation of the tracer in the caudate nucleus and putamen which contain the highest densities of dopamine receptors (the corpus striatum is the site of greatest concentration of dopamine receptors in normal patients). Thalamic and cerebellar activity is low. C-11 raclopride is more sensitive to changes in dopamine concentration as it binds D2 receptors more selectively and has negligible binding to serotonin receptors. The agent can be used to monitor relative changes in synaptic dopamine concentration.
An increased number of D2 receptors in the caudate and putamen can be found in patients with untreated schizophrenia, however, patients with decreased dopamine D2 receptor concentrations have also been described. Because neuroleptics bind to dopamine receptors, the amount of available dopamine receptors demonstrated on PET imaging will reflect the amount of bound ligand (ie: NMS activity will decrease as greater amounts of the neuroleptic agent is bound). Patients receiving adequate treatment with neuroleptic drugs have approximately 90% of their dopamine receptors occupied (implying a near total blockage of dopaminergic neurotransmission). It was hoped that PET imaging could be used to identify patients that might not respond to neuroleptic therapy. Unfortunately studies have not been able to demonstrate a difference in receptor blockade between responders and non-responders [9].
The lack of consistent PET findings in patients with schizophrenia would suggest that schizophrenia is a heterogeneous disorder with subgroups of patients with different cerebral abnormalities.
Substance Abuse:
11-C labeled cocaine shows a heterogeneous distribution within the brain, with maximal uptake in the basal ganglia. Studies have also demonstrated that cocaine produces decreased metabolism in the cortical and subcortical structures [9], as well as widespread abnormalities in cerebral blood flow [9]. These flow abnormalities are probably the result of cocaine induced vasoconstriction, which can lead to tissue ischemia and infarction if severe [9]. The perfusion abnormalities are mostly localized to the frontal cortex, and cerebellar flow is generally unimpaired.
The region most commonly reported to be abnormal in metabolic studies of alcoholics is the frontal cortex [9]. Other studies during alcohol intoxication have demonstrated more heterogeneous decreases in metabolism, with the areas greatest affected being the occipital cortex, the cerebellum, and the prefrontal cortex- these areas also contain the highest concentration of benzodiazepine receptors in the brain [9]. Other areas which have relatively low concentrations of these receptors, such as the basal ganglia and corpus callosum, demonstrate only minimal metabolic changes following alcohol consumption.
C-11 Carfentanyl has a high affinity for mu opiate receptors which mediate analgesia and respiratory depression. The highest concentration of opiate receptors exist in the thalamus (highest concentration of mu receptors), striatum (caudate nucleus), and amygdala. There are very low concentrations of opiate receptors in the motor-sensory and occipital cortex. The distribution of the tracer can be changed by the prior administration of naloxone (an opiate antagonist) which results in markedly decreased uptake of the agent. C-11 carfentanyl studies of patients with partial complex seizures have demonstrated increased opiate receptor binding in the temporal lobe containing the epileptic focus (corresponding to the area of decreased glucose utilization identified on FDG imaging).
REFERENCES:
(1) J Nucl Med 2001; Meltzer CC, et al. Does cerebral blood flow decline in healthy aging? A PET study with partial-volume correction. 41: 1842-1848
(2) J Nucl Med 2001; Fazio F, Perani D. Importance of partial-volume correction in brain PET studies. 41: 1849-1850 (No abstract available)
(3) J Nucl Med 2001; Hoffman JM, et al. FDG PET imaging in patients with pathologically verified dementia. 41: 1920-28
(4) J Nucl Med 2001; Silverman DHS, Phelps ME. Evaluating dementia using PET: How do we put into clinical perspective what we know to date? 41: 1929-32 (No abstract available)
(5) J Nucl Med 1993; Henry T, et al. Clinical evaluation of interictal fluoring-18-fluorodeoxyglucose PET in partial epilepsy. 34: 1892-1898
(6) J Nucl Med 2001; O'Brien TJ, et al. The utility of a 3-dimensional, large field-of-view, sodium iodide crystal-based PET scanner in the presurgical evaluation of partial epilepsy. 42: 1158-1165
(7) J Nucl Med 2001; Derdeyn CP, et al. Comparison of PET oxygen extraction fraction methods for the prediction of stroke risk. 42: 1195-1197
(8) Semin Nucl Med 1992; Mazziotta JC, et al. The use of positron emission tomography in the clinical assessment of dementia. 22: 233-246
(9) Semin Nucl Med 1992; Volkow ND, Fowler JS. Neuropsychiatric disorders: Investigation of schizophrenia and substance abuse. 22: 254-267
(10) Semin Nucl Med 1992; Broich K, et al. Positron emission tomography in cerebral vascular disorders. 22: 224-232
(11) Semin Nucl Med 1992; Chungani HT. The use of positron emission tomography in the clinical assessment of epilepsy. 22: 247-252
(12) J Nucl Med 2001; Huang WS, et al. Evaluation of early-stage Parkinson's disease with 99mTc-TRODAT-1 imaging. 42: 1303-1308
(13) J Nucl Med 2001; Feigin A, et al. Metabolic network abnormalities in early Huntington's disease: an [18F]FDG PET study. 42: 1591-1595
(14) J Nucl Med 2001; Herholz K, et al. Direct comparison of spatially normalized PET and SPECT scans in Alzheimer's disease. 43: 21-26
(15) J Nucl Med 2002; Silverman DH, et al. Evaluating early dementia with and without assessment of regional cerebral metabolism by PET: A comparison of predicted costs and benefits. 43: 253-266
(16) Radiology 2003; Petrella JR, et al. Neuroimaging and early diagnosis of Alzheimer disease: a look to the future. 226: 315-336
(17) JAMA 2001; Silverman DH, et al. Positron emission tomography in evaluation of dementia. 286: 2120-2127
(18) AJR 2004; Norfray JF, Provenzale JM. Alzheimer's disease: neuropathologic findings and recent advances in imaging. 182: 3-13
(19) Radiology 2004; Patwardhan MB, et al. Alzheimer disease: operating characteristics of PET- a meta-analysis. 231: 73-80
(20) J Nucl Med 2004; Silverman DH. Brain 18F-FDG PET in the diagnosis of neurodegenerative dementias: comparison with perfusion SPECT and with clinical evaluations lacking nuclear imaging. 45: 594-607