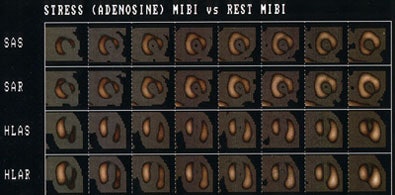
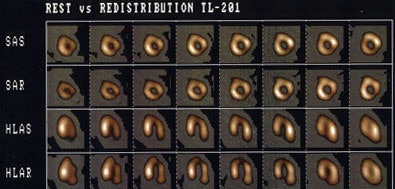
Myocardial Viability Imaging
Background
Patients with chronic coronary artery disease and severe left ventricular dysfunction (left ventricular ejection fractions below 30% to 35%) have poor survival. Patients with ejections fractions greater than 50% have a 4-year survival rate of 92%, compared to only 57% for those with ejection fractions below 35% [34]. It has been estimated that between 25-40% of patients with chronic coronary artery disease and left ventricular (LV) dysfunction have the potential for significant improvement in left ventricular function following revascularization [30]. In patients with left ventricular ejection fractions (LVEF's) below 30% and predominant heart failure symptoms, coronary revascularization can improve LV function, heart failure symptoms, and long-term prognosis when compared with medical treatment alone [25,30]. Unfortunately, patients with reduced LVEF's are at a greater risk for surgical mortality from coronary artery bypass graft (CABG) (about 4% mortality) and physicians are reluctant to recommend surgery if the prospects for benefit are limited.
Because post-operative improvement in LVEF has been related to the preoperative identification of viable myocardial tissue, viability is a crucial factor when considering chronic congestive heart failure (CHF) patients for coronary artery bypass grafting [30]. Patients with a greater percentage of viable tissue are more likely to have improved LVEF, decreased symptoms, and improved survival [25,49]. Patients with evidence of viability involving 18% or more of the LV myocardium have been shown to have the greatest improvement in functional status [59]. In a meta-analysis, patients with predominantly viable myocardium who were treated medically had a cardiac death rate of 16% per year [55]. Patients with predominantly viable myocardium who underwent revascularization had an 80% lower mortality rate and a cardiac event rate of only 3.2% per year [55]. In contrast, no difference in cardiac death rate was seen in patients with predominantly non-viable myocardium who underwent revascularization versus medical therapy (7.7% versus 6.2%, respectively) [55]. However, even patients that do not demonstrate an improvement in ejection fraction, can have a survival benefit following revascularization- possibly related to a reduced incidence of subsequent ischemic events [49]. Of course, the presence of viable myocardial tissue is just one factor to consider when evaluating patients for revascularization surgery. Although restoration of myocardial function is unlikely in the absence of viable tissue, revascularization can be associated with other benefits, such as relief of ischemia, pain relief, stabilization (or reversal) of remodeling, and decreased incidence of arrhythmias [59]. The bottomline is that there is "a body of literature demonstrating that patients with severe LV dysfunction after MI, severe angina (with or without heart failure), and bypassable coronary arteries show a survival benefit from revascularization compared to medical therapy alone" [49].
Immediately following revascularization there may be a period of stunning in which LV function deteriorates. Imaging 2 to 3 months after the procedure is probably more representative of true post-op LV function. Improvement in regional function may occur without improvement in overall ejection fraction. LV size is also an important determinant of the potential for recovery of function--little to no improvement is seen in patients with marked LV dilatation [5]. Terms which are used in the discussion of myocardial viability include stunned and hibernating myocardium.
Stunned myocardium
Stunned myocardium refers to myocardial contractile dysfunction that follows a period of transient ischemia (coronary occlusion), even after flow has been restored to an area that has no irreversible damage. Stunning represents a flow-contraction mismatch that is typically seen in the clinical setting of thrombolytic therapy or angioplasty/stenting for acute myocardial infarction. In these cases, the region of affected myocardium will appear perfused on nuclear imaging exams, but will demonstrate wall motion abnormalities on gated imaging or echocardiography. Stunning represents the combined results of ischemic and reperfusion injury. In the classical setting (transient coronary artery occlusion), stunning may persist for a few days to 8 weeks after revascularization [1], but general improvement in regional wall motion is seen after 2 to 3 weeks without further intervention.
Stunning can also be seen in the recovery phase after exercise induced ischemia (i.e.: after stress testing, gated exams may show wall motion abnormalities in regions of ischemic myocardium that do not persist on rest images). The time course of resolution of ischemic LV dysfunction following exercise has been reported to range from immediate to up to 2 hours [47]. Stunning is also seen in the post-operative CABG period, in the setting of unstable angina, and following coronary angioplasty [6]. Stunning has been reported with pharmacologic stress imaging [44]. In these cases it is most likely due to a "coronary artery steal" in which the increased blood flow in a normal artery siphons flow away from the myocardial tissue distal to a high grade stenosis via collateral channels (i.e.: the affected segment becomes truly ischemic) [44].
Proposed mechanisms for the observed wall motion abnormalities include:
- Abnormal energy utilization by myofibrils
- Production of cytotoxic oxygen free radicals
- Altered calcium flux
- Accumulation of neutrophils in previously ischemic tissue
Hibernating myocardium
Hibernating myocardium represents severely ischemic, but viable myocardial tissue. There is a chronic reduction in myocardial metabolism/contractility in order to match a long-standing decrease in blood supply (chronic ischemia). In other words, the cells maintain viability, but cannot perform mechanical work [20]. This may be a protective response by the myocytes to reduce their oxygen demand in the setting of reduced oxygen availability. However, this is a reversible phenomenon and ventricular dysfunction can improve if flow is restored. Evidence suggests that myocardial hibernation is an incomplete adaptive response to ischemia which cannot be maintained indefinitely and that once identified, prompt revascularization should be performed [49,59].
Another theory proposes that hibernating myocardium is the result of repetitive episodes of myocardial stunning which leads to chronic left ventricular dysfunction [54]. In some studies, the presence of normal resting blood flow in regions of hibernating myocardium, but decreased flow reserve supports this theory [54].
The presence of hibernating myocardium in a patient with LV dysfunction indicates a high risk for subsequent cardiac events when treated with medical therapy alone [49]. Because enhanced left ventricular function after revascularization is associated with improved survival, it is crucial to identify areas of viable myocardial tissue (hibernating myocardium).
The potential for improved function following revascularization is dependent upon many variables- of which the presence of viable myocardial tissue is of obvious importance. However, other factors such as the timing of revascularization after the onset of hibernation may influence the degree of recovery following cardiac surgery [52]. In areas of hibernating myocardium there is progressive cellular degeneration over time which may reduce the chance for complete functional recovery if revascularization is delayed [52]. Also- the presence and magnitude of stress-induced ischemia, the degree of left ventricular remodeling, and the adequacy of target coronary vessels can all affect functional outcome [57].
On gated SPECT imaging or echocardiography areas of hibernating myocardium can be expected to demonstrate a wall motion abnormality.
Thallium imaging in the identification of hibernating myocardium:
Rest-redistribution thallium imaging is used to assess for the presence of viable myocardial tissue with severely diminished perfusion (hibernating myocardium). A standard stress-rest-reinjection examination can provide similar information, as well as evaluate for the presence of ischemia. Stress-redistribution thallium or dual-isotope exams using a rest thallium injection may not be as useful, as between 40-50% of fixed thallium defects on non-reinjection images have been shown to be viable on FDG imaging.
Technique:
A rest-redistribution exam is useful when the clinical question pertains exclusively to the presence or absence of myocardial viability and not the detection of inducible ischemia. The exam is performed with a rest 3 to 4 mCi injection of thallium. Images should be acquired at the time of injection and 3 to 4 hours later. Reinjection prior to 4-hour delayed imaging is not performed and 24-hour delayed images are not generally required.
Findings:
Three types of abnormalities can be identified:
- Clearly reversible abnormalities: Rest perfusion defects which normalize on delayed images are indicative of severely ischemic, but viable myocardium (hibernating myocardium). Patients with areas of myocardial viability and left ventricular systolic dysfunction have been shown to have a much better prognosis when treated with surgical revascularization as opposed to medical therapy [36].
- Fixed defects with severely decreased tracer activity: Rest perfusion defects that do not demonstrate improved activity on delayed images most likely represent scar, although a small percentage (up to 20%) show improved regional wall motion following revascularization [37]. General, defects with less than 50% of peak activity are felt to represent scar (or more extensive non-transmural MI) with a low likelihood for improved LV function after revascularization. Note: For fixed defects with activity less than 50% of the maximal uptake, up to 25% have been shown to be viable by FDG imaging [38].
- Fixed defects with mild to moderate decreased tracer activity: About 50% of these regions show improved wall motion following revascularization. These areas may contain mixed viable myocardial tissue and scar (i.e.: regions of non-transmural myocardial infarction). A mild defect (less than 30% reduction in activity) is indicative of viability with a better likelihood for improvement in wall motion following revascularization. Other authors suggest that a fixed defect with counts of 50% or greater of the peak activity indicates myocardial viability.
Sensitivity and specificity of rest thallium imaging:
See also FDG PET imaging for myocardial viabiliy
Preserved thallium uptake in regions of myocardium with dysfunctional wall motion is predictive of functional recovery after revascularization. The sensitivity of rest-redistribution thallium for the determination of viability and improvement in wall motion is between 67% to 84%, and the specificity is between 55% to 77% [11,48,51,53]. The positive and negative predictive accuracies are 69-72% and 70-92%, respectively, for the determination of recovery of regional left ventricular dysfunction following revascularization [20,35]. The sensitivity and specificity for the exam will vary depending on what percentage of maximal uptake is used to determine viability [40]- generally, segments with greater than 50% of peak activity are considered viable. Quantitative analysis of rest-redistribution images can aid in exam interpretation. Overall, thallium imaging is felt to have a high sensitivity for the identification of viable myocardial tissue, but a lower specificity [51]. Because the positive predictive accuracy of the exam is generally lower, it may lead to unnecessary revascularization [48].
When compared to PET viability imaging, a large percentage of mild to moderate thallium defects (with over 50 to 65% of maximal activity) on rest-redistribution images have been confirmed to be metabolically active on PET studies. Unfortunately, rest-redistribution thallium imaging can underestimate viability. In severe defects with less than 50% maximal uptake, metabolic activity has still been identified in up to half of these areas on PET imaging [41]. Rest-redistribution thallium imaging is slightly less accurate than PET imaging (PET perfusion-metabolism imaging has a positive predictive value and negative predictive values of 76-83% and 84-92%, respectively [20, 35]).
If there are no contraindications to stress testing a stress-rest-reinjection exam provides a more comprehensive assessment of the extent and severity of coronary artery disease (CAD) by demonstrating regional myocardial ischemia, without compromising information on myocardial viability [39].
Combined use of rest thallium imaging and dobutamine echocardiography (which evaluates contractile reserve) has also been evaluated for the assessment of myocardial viability [48,51,53]. The combined use of these exams may provide more accurate information than either technique independently [48,53], particularly in patients with an intermediate likelihood of viability by either test [51].
Reverse-Redistribution on rest-redistribution exams:
On rest-redistribution imaging two patterns of reverse-redistribution can be identified. In one pattern there is normal uptake on the rest image, but a defect is identified on the redistribution exam. This finding suggests a high grade stenosis in the vessel which supplies that segment. It is also frequently (up to 40% of cases) associated with impaired myocardial contractile function in the affected segment, but improvement in wall motion is very likely following revascularization.
In the other pattern, there is a defect which is present on the rest image, but the defect demonstrates significantly decreased activity on the redistribution exam. Wall motion defects in these regions are more common (up to 60%), typically severe, and recovery of wall motion abnormalities following revascularization is very unlikely. Segments which demonstrate this finding should be classified as regions of scarring [42,43].
Technetium agent imaging of hibernating myocardium and myocardial viability
Thallium imaging with reinjection is superior to Tc-Sestamibi (and likely Tc-tetrofosmin) imaging in identifying viable myocardium in patients with chronic coronary artery disease [7]. Since the primary determinant of technetium perfusion agent uptake is regional coronary blood flow rather than tissue viability, technetium agents can underestimate the presence of viable myocardium in states of greatly reduced perfusion [57]. Approximately 35 to 60% of reversible myocardial regions on Thallium reinjection imaging will appear as fixed defects on exercise-rest images with Tc-Sestamibi [7,8]. Even if defect severity is taken into account, Tc-Sestamibi studies are likely to prove ineffective in separating subendocardial infarction from hibernating myocardium [9].
Generally, the likelihood for the presence of viable myocardium on technetium imaging is determined by the severity of the fixed perfusion defect. A moderate reduction in activity (51 to 85% of normal) in a fixed defect is more suggestive of viable myocardium and improves concordance with thallium imaging. Uptake within a defect of over 55% of the maximum cardiac activity is associated with a 70% likelihood of improved regional wall motion after revascularization [46]. Regions with uptake less than 55% peak activity have a lower likelihood of demonstrating improved wall motion after revascularization [46]. Quantitative analysis has improved sensitivity for the detection of viability compared to visual analysis [50]. In fact, a substantial number of dysfunctional segments with no evidence of viability at visual analysis exhibit preserved tracer uptake at quantitative analysis and improved wall motion following revascularization [50].
In another study based upon comparison with PET FDG imaging, Tc-sestamibi uptake of less than 30% of the peak myocardial activity had a predictive value of over 80% that this area represented a scar [10]. Unfortunately, 25% of segments with defects of 41% of peak activity, and 50% of segments with defects of 60% of peak activity were shown to be viable on FDG imaging [10]. Therefore, rest perfusion sestamibi defects with an uptake between 40%-60% of peak activity are intermediate probability for functional improvement following revascularization and further evaluation may be needed to make an appropriate clinical decision in these cases [50]. In general, segments with severe Tc-sestamibi defects with preserved FDG uptake were associated with severe hypokinesis on wall motion studies [10].
Attempts to improve the detection of viable myocardial tissue with technetium imaging agents include rest exams performed following the administration of nitrates (most likely the result of the vasodilatory effects of the nitrates) [11,12,56]. After nitrate administration, an increase in regional sestamibi uptake of 10% or greater predicts post-revascularization functional improvement [58]. Although concordance with FDG imaging has been shown to improve with the use of nitrates [56], the results are still inferior to FDG PET imaging [58]. Delayed redistribution cardiac imaging after resting Tc-MIBI injection has also been investigated. Such delayed images have revealed improved uptake of tracer in 24 to 38% of rest defects [13,14] and better concordance with PET viability imaging [56].
Despite these efforts to demonstrate the value of technetium agents, it must be remembered that reinjection thallium imaging (as discussed above) provides information regarding myocardial viability similar to that of PET imaging. The concordance between the two techniques regarding the presence of viable or nonviable myocardium is about 90%.
Tc-Sestamibi versus rest thallium for the detection of hibernating myocardium: The Tc-MIBI exam (top) demonstrates severe fixed perfusion defects involving the apex, anteroapex, and inferolateral wall. Because the patient had a severely reduced ejection fraction and was a candidate for revascularization, a rest thallium exam was performed to assess for the presence of viable myocardial tissue. The thallium exam (below) reveals extensive viable myocardial tissue in the regions of fixed Tc-MIBI defects. Thallium is superior to technetium agents for the assessment of myocardial viability. |
|
FDG PET imaging of hibernating myocardium
MR Imaging for Hibernating Myocardium:
MR imaging is also being studied for the assessment of myocardial viability [34]. Because of the excellent anatomic resolution that can be achieved with rapid cardiac imaging sequences, MR determination of viability is based on visible changes in myocardial wall thickness [34] or through the use of delayed contrast enhanced imaging .
For functional MR imaging, six to eight short axis sections can be obtained and images reviewed in cine format [34]. Hypokinetic or akinetic segments demonstrate less than 1 mm of wall thickening [34]. Normal myocardial tissue has an end-diastolic wall thickness greater than 5.5 mm and systolic wall thickening greater than 1.5 mm [34]. Hibernating myocardium has an end-diastolic wall thickens greater than 5.5 mm and wall thickening less than 1.5 mm [34]. Infarcted myocardium has an end-diastolic wall thickness less than 5.5 mm and wall thickening less than 1.5 mm [34].
Patients with acute myocardial infarctions less than 3 weeks old should not be evaluated for viability as areas of stunned myocardium may not have regained full function and the areas of infarcted myocardium may not have had time to allow the scarring process to produce wall thinning [34].
MR has a sensitivity of between 55% in predicting improvement in function in a myocardial segment after revascularization [34]. Segments felt to be representative of hibernating myocardium that failed to demonstrate improved function were commonly adjacent to infarcted segments or had nontransmural wall thinning [34]. The lack of improved function in regions adjacent to areas of infarcted myocardium may be related to mechanical tethering [34].
Also, myocardium adjacent to areas of chronic infarction have hypertrophied myocytes that have impaired contractile function [34]. In regions where wall thickness is greater than 5.5 mm, but less than normal, there is a reduction in muscle mass and the amount of recovery can be reduced. A subendocardial infarct with some preserved end diastolic wall thickening (EDWT) may limit improvement in left ventricular function [34].
Delayed contrast enhanced MR (DE MR) imaging is very sensitive for detecting areas of myocardial infarction and scar. For the MR exam delayed inversion-recovery fast low-angle shot contrast-enhanced imaging is performed in the short axis plane 15 minutes following the IV administration of 0.15 mmol of gadolinium per kg of body weight [60]. Areas of myocardial infarction (both acute and chronic) demonstrate late contrast enhancement in these regions following the administration of gadolinium (most likely secondary to edema and an increase in the extracellular space at the site of infarction) [60]. The transmural extent of hyperenhancement within the 1st week after MI can be used to predict late improvement in contractile function [60]. Regions with greater transmural enhancement are less likely to demonstrate improved wall motion [60]. Segments with infarct involving less than 25% of the wall thickness are more likely to recover function following revascularization [63]. DE MR has been shown to be superior to SPECT imaging for the detection of acute MI- particularly for inferior infarcts [61].
By about 30 days following the event, an area of infarction will demonstrate thinning of the myocardium and decreased signal on T2 images or gradient echo images due to the presence of fibrosis.
REFERENCES:
(1) J Nucl Med 1993; Schulman DS, et al. Right ventricular thallium-201 kinetics in
pulmonary hypertension: relation to right ventricular size and function. 34: 1695-700
(2) New Engl J Med 1993; Zaret BL, Wackers FJ. Nuclear cardiology (1). 329: 775-783
(3) Am J Cardiol 1990; Villanueva FS, et al. Prevalence and correlates of increased lung/heart ratio of thallium-201 during dipyridamole stress imaging for suspected coronary artery disease. 66: 1324-28
(4) Am.J.Cardiol 1990; Lette J, et al. Transient left ventricular cavitary dilation during dipyridamole-thallium imaging as an indicator of severe coronary artery disease. 66:1163-70
(5) J Nucl Med 1994; Iskandrian AS, et al. When is myocardial viability an important clinical issue? 35 (Suppl): 4S-7S
(6) J Nucl Med 1994; Leavitt JI, et al. Demonstration of viable, stunned myocardium with technetium-99m-sestamibi. 35: 1805-07
(7) J Nucl Med 1992; Cuocolo A, et al. Identification of viable myocardium in patients with chronic coronary artery disease: comparison of thallium-201 scintigraphy with reinjection and technetium-99m-methoxyisobutyl isonitrile. 33: 505-511
(8) Circulation 1994; Dilsizian V, et al. Myocardial viability in patients with chronic coronary artery disease. Comparison of 99mTc-sestamibi with thallium reinjection and [18F] fluorodeoxyglucose. 89: 578-87
(9) Radiol Clin North Am 1993; Kiat H, et al. Myocardial perfusion imaging using technetium-99m radiopharmaceuticals. 31: 795-815
(10) J Nucl Med 1994; Altehoefer C, et al. Significance of defect severity in technetium-99m-MIBI SPECT at rest to assess myocardial viability: comparison with fluorine-18-FDG PET. 35: 569-74
(11) J Nucl Med 1995; Maurea S, et al. Enhanced detection of viable myocardium by technetium-99m-MIBI imaging after nitrate administration in chronic coronary artery disease. 36: 1945-52
(12) J Nucl Med 1995; Bisi G, et al. Technetium-99m-sestamibi imaging with nitrate infusion to detect viable hibernating myocardium and predict postrevascularization recovery. 36: 1994-2000
(13) J Nucl Med 1995; Maurea S, et al. Myocardial viability index in chronic coronary artery disease: technetium-99m-methoxy isobutyl isonitrile redistribution. 36: 1953-60
(14) Circulation 1994; 89: 578-87
(15) J Nucl Med 1998; Bax JJ, et al. Comparison of Fluorine-18-FDG with rest-redistribution thallium SPECT to delineate viable myocardium and predict functional recovery after revascularization. 39: 1481-1486
(16) J Nucl Med 1995; Ohte N, et al. Clinical significance of reverse redistribution on
24-hour delayed imaging of exercise thallium-201 myocardial SPECT: comparison with
myocardial fluorine-18-FDG-PET imaging and left ventricular wall motion.
36: 86-92
(17) J Nucl Med 1993; Liu P, Burns RJ. Easy come, easy go: time to pause and put thallium reverse redistribution in perspective. 34: 1692-94
(18) J Nucl Med 1995; Soufer R, et al. Relationship between reverse redistribution on planar thallium scintigraphy and regional myocardial viability: a correlative PET study.36: 180-87
(19) Nucl Med Annual 1993; Parmett SR, Ongseng. Myocardial perfusion imaging: Pifalls and pearls. Ed. Freeman LM. Raven Press, NY. 195-221
(20) Radiographics 1999; Jadvar H, et al. SPECT and PET in the evaluation of coronary artery disease. 19: 915-926
(21) AJR 2000; Robinson VJB, et al. Causes of transient dilatation of the left ventricle during myocardial perfusion imaging. 174: 1349-1352
(22) Semin Nucl Med 1997; Kataoka T. False-positive myocardial perfusion scintigraphy in syndrome X. 27 (2): 186-189
(23) J Am Coll Cardiol 1996; Mazzanti M, et al. Identification of severe and extensive coronary artery disease by automatic measurement of transient ischemic dilatation of the left ventricle in dual-isotope myocardial perfusion SPECT. 27: 1612-1620
(24) J Nucl Med 2000; Verna E, at al. "False-positive" myocardial perfusion scintigraphy findings in patients with angiographically normal coronary arteries: Insights from intravascular sonography studies. 41: 1935-1940
(25) J Nucl Med 2001; Bax JJ, et al. Relationship between preoperative vaiability and postoperative improvement in LVEF and heart failure symptoms. 42: 79-86
(26) J Nucl Med 2001; Tawakol A, Gewirtz H. Does CABG improve left ventricular ejection fraction in patients with ischemic cardiomyopathy, and does it matter? 42: 87-90 (No abstract available)
(27) J Nucl Med 2001; Matsumoto N, et al. Quantitative assessment of motion artifacts and validation of a new motion correction program for myocardial perfusion SPECT. 42: 687-694
(28) J Nucl Med 1993; Prigent FM, et al. Effect of motion on thallium-201 SPECT studies: a simulation and clinical study. 34:1845-50
(29) J Am Coll Cardiol 1997 Bax JJ, et al. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: Pooled comparison data. 30: 1451-60
(30) Semin Nucl Med 2000; Bas JJ, et al. 18-Fluorodeoxyglucose imaging with positron emission tomography and single photon emission computed tomography: Cardiac applications. 30: 281-298
(31) Semin Nucl Med 1995; Newhouse HK, Wexler JP. Myocardial perfusion imaging for evaluating interventions in coronary artery disease. 25: 15-27
(32) J Nucl Med 2001; Tanaka R, Nakamura T. Time course evaluation of myocardial perfusion after reperfusion therapy by 99mTc-tetrofosmin SPECT in patients with acute myocardial infarction. 42: 1351-1358
(33) J Nucl Med 2001; Harel F, et al. Clinical impact of combination of scatter, attenuation correction, and depth-dependent resolution recovery for 201Tl studies. 42: 1451-56
(34) Radiology 2001; Oshinski JN, et al. Quantitative prediction of improvement in cardiac function after revascularization with MR imaging and modeling: Initial results. 221: 515-522
(35) J Nucl Med 1994; Maddahi J. Role of thallium-201 and PET imaging in evaluation of myocardial viability and management of patients with coronary artery disease and left ventricular dysfunction. 35: 707-15
(36) Chest 1999; Morse RW, et al. Rest-redistribution 201-Tl single-photon emission CT imaging for determination of myocardial viability. 115: 1621-1626
(37) Circulation 1993; Ragosta M, et al. Quantitative planar rest-redistribution 201Tl imaging in detection of myocardial viability and prediction of improvement in left ventricular function after coronary bypass surgery in patients with severely depressed left ventricular function. 87: 1630-41
(38) J Nucl Med 1995; Burt RW, et al. Direct comparison of fluorine-18-FDG SPECT, fluorine-18-FDG PET and rest thallium-201 SPECT for detection of myocardial viability. 36: 176-79
(39) Circulation 1993; Dilsizian V, Bonow RO. Current diagnostic techniques of assessing myocardial viability in patients with hibernating and stunned myocardium. 87: 1-20
(40) (7) J Nucl Med 1998; Pace L, et al. Identification of viable myocardium in patients with chronic coronary artery disease using rest-redistribution thallium-201 tomography: Optimal image analysis. 39: 1869-1874
(41) J Nucl Med 1994; Hendel RC. Single-photon perfusion imaging for the assessment of myocardial viability. 35:(Suppl.): 23S-31S
(42) J Nucl Med 1993; Pace L, et al. Reverse redistribution in resting thallium-201 myocardial scintigraphy in patients with coronary artery disease: relation to coronary anatomy and ventricular function. 34: 1688-92
(43) J Nucl Med 1995; Pace L, et al. Reverse redistribution in resting thallium-201 myocardial scintigraphy in chronic coronary artery disease: an index of myocardial viability. 36: 1968-73
(44) J Nucl Cardiol 2001; Dakik HA, Alam S. Myocardial stunning induced and detected by adenosine stress perfusion imaging. 8: 711-2 (No abstract available)
(45) J Nucl Med 2002; Sciagra R, et al. Comparison of dobutamine echocardiography and 99mTc-sestamibi tomography for prediction of left ventricular outcome after acute myocardial infarction treated with successful primary coronary angioplasty. 43: 8-14
(46) J Nucl Cardiol 2002; Acampa W, et al. Tetrofosmin imaging in the detection of myocardial viability in patients with previous myocardial infacrtion: comparison with sestamibi and Tl-201 scintigraphy. 9: 33-40
(47) J Nucl Med 2002; Yamagishi H, et al. Incremental value of left ventriuclar ejection fraction for detection of multivessel coronary artery disease in exercise 201Tl gated myocardial perfusion imaging. 43: 131-139
(48) J Nucl Cardiol 2002; Dellegrottaglie S, et al. Prediction of the long term effects of revascularization on regional and global left ventricular function by dobutamine echocardiography and rest Tl-201 imaging alone and in combination in patients with chronic coronary artery disease. 9: 174-82
(49) J Nucl Cardiol 2002; Di Carli MF. Assessment of myocardial viability after myocardial infarction. 9: 229-235
(50) J Nucl Cardiol 2002; Acampa W, et al. Quantification of SPECT myocardial perfusion imaging. 9: 338-42
(51) J Nucl Med 2002; Bax JJ, et al. Sequential 201Tl imaging and dobutamine echocardiography to enhance accuracy of predicting improved left ventricular ejection fraction after revascularization. 43: 795-802
(52) J Nucl Med 2002; Matsunari I, et al. Sequential strategy using multimodality viability tests: does it work? 43: 803-805
(53) J Nucl Cardiol 2003; Piscione F, et al. Relationship between contractile reserve, Tl-201 uptake, and collateral angiographic circulation in collateral-deoendent myocardium: implications regarding the evaluation of myocardial viability. 10: 17-27
(54) J Nucl Cardiol 2003; Nowak B, et al. Assessment of myocardial viability in dysfunctional myocardium by resting myocardial blood flow determined with oxygen 15 water PET. 10: 34-45
(55) J Nucl Cardiol 2003; Beller GA. Clinical value of myocardial perfusion imaging in coronary artery disease. 10: 529-542
(56) J Nucl Cardiol 2003; He W, et al. Tc-99m tetrofosmin tomography after nitrate administration in patients with ischemic left ventricular dysfunction: relation to metabolic imaging by PET. 10: 599-606
(57) J Nucl Cardiol 2003; Di Carli MF. The quest for myocardial viability: is there a role for nitrate-enhanced imaging? 10: 696-699
(58) J Nucl Cardiol 2004; Giorgetti A, et al. Baseline/postnitrate Tc-99m tetrofosmin mismatch for the assessment of myocardial viability in patients with severe left ventricular dysfunction: comparison with baseline Tc-99m testrofosmin scintigraphy/FDG PET imaging. 11: 142-51
(59) J Nucl Cardiol 2004; Udelson JE, et al. The historical and conceptual evolution of radionuclide assessment of myocardial viability. 11: 318-334
(60) Radiology 2003; Kitagawa K, et al. Acute myocardial infarction: myocardial viability assessment in patients early thereafter- comparison of contrast-enhanced MT imaging with resting 201Tl SPECT. 226: 138-144
(61) Radiology 2004; Lund GK, et al. Acute myocardial infarction: evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. 232: 49-57
(62) J Nucl Cardiol 2004; Maddahi J. Factors influencing predictive value of FDG imaging for evaluating myocardial viability. 11: 526-526
(63) Circulation 2001; Choi KM, et al. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. 104: 1101-1107