Asbestosis:
View images of asbestosis and asbestos related lung disease
Clinical:
The diagnosis of "asbestosis" requires both a history of asbestos exposure and evidence of interstitial lung disease. "Asbestos related disease" is the term used to describe other effects of asbestos on the lungs or pleura. Pleural changes are seen in 90% of patients with a history of asbestos exposure. Other pulmonary complications of asbestos exposure include: Round atelectasis, bronchogenic carcinoma, and malignant mesothelioma.
- Bronchogenic carcinoma is the most common neoplastic complication of asbestos exposure and its occurrence may be dose related. Lung cancer is estimated to develop in 20-25% of workers who are heavily exposed to asbestos [9]. Cigarette smoking increases the risk for lung cancer 90 times. Adenocarcinoma is the most common cell type.
- Mesothelioma: There is an increased risk for both pleural or peritoneal mesothelioma which is not dose related and there is no increased risk for this lesion in smokers. There is typically a 20 to 40 year latent period prior to the development of the tumor [9]. The risk of mesothelioma in an asbestos worker is approximately 10% over their lifetime [9]. See also "Mesothelioma"
- An increased risk for non-pulmonary neoplasm's is also seen including: Gastric carcinoma- possibly due to particles being coughed-up and swallowed and bladder carcinoma.
Asbestos fibers can be subdivided into two large groups- amphiboles (stiff and straight) and serpantines (curly and flexible) [10]. The amphiboles asbestos fiber "crocidolite" has the greatest association with eliciting mesothelioma and bronchogenic carcinoma. There is only one serpentine fiber "Chrysotile," which accounts for approximately 80-90% of the asbestos used today. Chrysotile tends to be deposited in the proximal airways, is relatively water soluble, and has a strong association with the formation of pleural plaques (chrysotile fiber fragments have been identified in as many as 50% of plaques at electron microscopy [14]). It is postulated that exposure to pure chrysotile does not cause malignant mesothelioma, however, contaminants such as tremolite (an amphibole fiber) may be responsible for tumor development in these individuals.
Asbestos fibers produces a non-specific interstitial fibrosis (asbestosis) and there is a definite dose-effect relationship [9,10]. The lag between exposure and onset of symptoms is usually 20 years or longer [10]. The microscopic hallmark of asbestos exposure is the asbestos body or "ferruginous bodies" which results from the deposition of iron-protein complexes on an asbestos fiber core. The structures have a beaded rod-like appearance. Macrophages are not disrupted by the asbestos fiber as they are by silica. Rather, the fibers elicit a cellular inflammatory response which leads to fibrosis. Magnesium silicate (a salt of silicic acid) is believed to be the component of asbestos that elicits the inflammatory response (1).
X-ray:
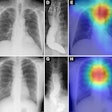
On plain small, irregular opacities (or a prominent interstitial pattern) are identified predominantly in a lower lobe distribution. The heart border will become shaggy. This fine reticular pattern eventually progresses to a coarse linear pattern. In the late stages of the disease there is honey-comb lung and volume lose. CT will detect calcification in up to 60% of the pleural plaques.
HRCT can suggest changes due to asbestosis in one third of patients with a normal CXR, however, a normal CT cannot exclude asbestosis. Abnormalities due to asbestosis are usually bilateral and somewhat symmetric. On HRCT the primary site of parenchymal involvement is the posterior, subpleural region of the lower lobes. As such, patients should be imaged in the prone position at maximal inspiration. CT findings in asbestosis include: 1- "Dot-like" opacities in the sub-pleural lung several millimeters from the pleura. This is the earliest abnormal finding recognizable on HRCT and it is due to peribronchiolar fibrosis extending to involve the contiguous airspaces and alveolar interstitium. 2- Thickened subpleural lines occur due to interlobular septal (irregular thickening), and intralobular fibrosis in the lung periphery; 3- Parenchymal bands: Non-tapering linear densities measuring 2 to 5 cm in length that contact the pleural surface are probably most commonly identified in patients with asbestos related lung disease. Multiple parenchymal bands were seen in up to 66-76% of patients and have a basal predominance. Because of their association with sites of pleural thickening, they are felt to represent the earliest stages of rounded atelectasis; 4- Honeycombing: Small, 2 to 5 mm cystic spaces with discrete thickened walls. This represents the non-specific end stage of many fibrosing conditions and it is found in nearly 100% of patients with end-stage asbestosis (2). 5- Curvilinear subpleural lines and hazy subpleural densities: Curvilinear lines are located within 1 cm of the periphery and course parallel to the chest wall. Patchy areas of increased density may be seen normally in the dependent lung, they are only abnormal when they persist in non-dependent lung. Subpleural lines have been identified in up to 40% of patients with asbestosis (however, subpleural lines have been demonstrated in 15-20% of normal patients). Although the peripheral interstitial pattern seen in asbestosis has also been described in other disorders, its demonstration in patients with a strong history of asbestos exposure offers compelling evidence for the diagnosis of asbestosis. Ground-glass opacity is uncommon in patients with asbestosis.
CT is considered the most accurate means for detecting asbestos related pleural abnormalities. HRCT has been shown to be as sensitive as conventional CT in the detection of pleural plaques, despite the use of selective sampling.
Non-malignant asbestos related pleural disease includes:
1- Pleural effusions: Between 3 to 5% of asbestos workers develop "benign asbestos pleurisy." Recurrent pleural effusions may occur in first decade post exposure and are generally small and unilateral. They are due to a pleuritis elicited by the asbestos particles. About two-thirds of patients are asymptomatic. Those with symptoms complain of chest pain, pleuritis [13], fever [13], or less commonly dyspnea. The effusion is exudative and serosanguinous in 45%, serous in 38%, and frankly bloody in 17% of patients. Although the exudate may be hemorrhagic, the patients do not have an underlying malignancy. Asbestos bodies are seldom or never found in the pleural fluid [13]. Nearly half of the affected patients will develop diffuse pleural thickening as the effusion resolves. [3]
2- Pleural plaques: These are the most common manifestation of asbestos exposure and are seen in 20 to 60% of heavily exposed individuals [14]. There is about a 20 year latent period before their development and they are not pre-malignant [14]. The plaques are bilateral (75%), usually asymmetric, and stable over time. Calcifications are identified in 10-20% on plain film and up to 60% by CT. The plaques originate on the parietal pleura, but may rarely be seen in the visceral pleura in the interlobular fissures. Visceral plaques are associated with abnormality in the adjacent lung parenchyma- including short interstitial lines that radiate from the plaque ("hairy plaque") [10]. Most pleural plaques occur in the posterolateral lower half of the thorax (diaphragmatic dome and posterior and paraspinal regions below the level of the sixth ribs) and tend to spare the apices and costophrenic angles [14]. The lesions also occur on the central tendinous portion of the diaphragm. The mediastinal pleura is less commonly affected, but may be detected in up to 40% of patients by CT. Subtle thickening can be reliably diagnosed when it is visible at more than one level, the thickened pleural indents the subadjacent lung, and extrapleural fat separates the thickened pleura from the chest wall.
3- Pleural calcification (25-40% of plaques have detectable calcification on plain film). A calcified plaque on the diaphragm is virtually pathognomonic for prior asbestos exposure [13].
4- Diffuse Pleural Fibrosis: Diffuse pleural fibrosis (DPF) is rare complication of asbestos exposure and is characterized by a diffuse pleural thickening which encases the lung. It may follow an asbestos related effusion and is associated with significant impairment in pulmonary function. The thickening measures from 2 to 10 mm (but at least 5 mm in one site [10]); extends more than 8 cm in the craniocaudad direction and 5 cm in width (or cover between 25% to 50% of the total chest wall [10]); may contain (rarely [10]) calcification; and there may be associated thickening of the subpleural fat. DPF does not demonstrate the nodularity seen with mesothelioma. The condition also spares the mediastinum, but does involve the interlobar fissures [10]. It can be bilateral [10].
5- Round atelectasis: Round atelectasis represents folded atelectatic lung with fibrous bands and adhesions to the visceral pleura. There is a high incidence in asbestos workers (most likely due to high degree of pleural disease). Over 80% of cases of rounded atelectasis are associated with asbestos. Affected patients are typically asymptomatic and the mean age at presentation is 60 years. It is seen most commonly posteriorly in the lung bases (90%), especially in the paraspinal gutter. Over time, most lesions remain stable in size, but enlargement or regression have been described. Three criteria are necessary for the diagnosis of rounded atelectasis: 1- A focal mass-like lesion abutting a region of thickened pleura; 2- Associated volume loss in the involved lobe; and 3- Bronchovascular structures radiate from the lesion in a "comet tail" fashion towards the hilum. Round atelectasis can co-exist with malignant mesolthelioma which will appear as a pleural mass frequently associated with a pleural effusion (pleural effusion is typically absent or minimal with round atelectasis) [6].
Radiograph: CXR demonstrates a crescentic/elliptical soft tissue mass abutting the pleural surface associated with evidence of pleural disease (thickening or calcification). The mass typically forms an acute angle with the pleural confirming it's intrapulmonary location.
Computed Tomography: On CT the pleural thickening or calcification is clearly identified. Thickened lung markings and vessels swirl into the lesion which abuts the pleural surface ("comet tail sign"- central bronchovascular pedicle)- however, this finding may be absent in up to 8% of cases. Curvilinear air bronchograms are often visible within round atelectasis. The lesion is very commonly based or tethered by a strand to a thickened pleural surface. Pericicatril emphysema may also be seen. Volume loss within the affected lung is also common- often characterized by displacement of the fissure bounding the affected lobe. Calcification may also be found within areas of round atelectasis (up to 25% of cases)- possibly secondary to engulfment of a granuloma within the atelectatic lung, or invagination of a calcified pleural plaque. The lesion will enhance homogeneously after the administration of I.V. contrast (up to nearly 90% of cases). Inhomogeneous enhancement is atypical for rounded atelectasis [12].
Despite the ability of computed tomography to accurately identify features which are often associated with round atelectasis, it should be remembered, that the findings described above are not necessarily completely diagnostic for this entity. Other lesions can mimic these findings and rarely lung cancer can co-exist in an area of round atelectasis [12].
Magnetic Resonance Imaging: On MRI, rounded atelectasis has a higher signal intensity than adjacent pleura on T1 images, and the folded visceral pleura may sometimes be identified as a curved, low-signal line within the atelectatic lung tissue. [4,5]
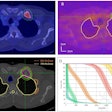
PET: Studies indicate that round atelectasis is not metabolically active on FDG-PET imaging [7].
|
Round atelectasis on PET FDG imaging: The images below demonstrate the typical CT and FDG PET findings in round atelectasis. On CT, there are thickened lung markings and vessels which swirl into the lesion that abuts an area of pleural thickening. Note the lack of metabolic activity in round atelectasis on PET imaging. |
|
|
REFERENCES:
(1) Chest Radiology, 3d ed., Reed, p.237-38
(2) HRCT of the Lungs, 2nd ed., p.56
(3) ACR Syllabus #40:p.500-501
(4) J Thorac Imag 1996; 11:p. 187-197
(5) J Thorac Imag 1997; 12: 54-58
(6) AJR 1998; Munden RF, et al. Rounded atelectasis and mesothelioma. 170: 1519-1522
(7) J Computer Assit Tomogr 1998; McAdams HP, et al. Evaluation of patients with round atelectasis using 2-[18-F] Fluoro-2-deoxy-D-glucose PET. 22(4): 601-604
(8) Radiology 1999; Partap VA. The comet tail sign. 213: 553-554 (No abstract available)
(9) Radiographics 2001; Kim KI, et al. Imaging of occupational lung disease. 21: 1371-1391
(10) Radiographics 2002; Roach HD, et al. Asbestos: when the dust settles- an imaging review of asbestos-related disease. 22: S167-S184
(11) AJR 2003; Akira M, et al. High-resolution CT of asbestosis and idiopathic pulmonary fibrosis. 181: 163-169
(12) AJR 2004; Nakazono T, et al. Squamous cell carcinoma coexisting in rounded atelectasis: diagnostic pitfalls. 182: 79-80
(13) Chest 2004; Cugell DW, Kamp DW. Asbestos and the pleura. 125:
1103-1117
(14) Radiographics 2012; Walker CM, et al. Tumorlike conditions of
the pleura. 32: 971-985