Researchers from Harvard University have developed a new 3D bioprinting method that uses a combination of stem cells and 3D CT data to produce functional, patient-specific organ tissue. The resulting 3D-bioprinted models are poised to facilitate organ repair and restoration, according to an article recently published online in Science Advances.
The proprietary technique, called sacrificial writing into functional tissue (SWIFT), is a multistep biomanufacturing process that involves creating organ building blocks composed of hundreds of thousands of induced pluripotent stem cells and then rapidly 3D bioprinting vasculature into those building blocks.
The group used the SWIFT technique to create cardiac tissue that was perfusable and also beat consistently for a period of seven days (Sci Adv, September 6, 2019, Vol. 5:9, eaaw2459).
"This is an entirely new paradigm for tissue fabrication," co-lead author Mark Skylar-Scott, PhD, said in a statement from the university's Wyss Institute. "Rather than trying to 3D print an entire organ's worth of cells, SWIFT focuses on only printing the vessels necessary to support a living tissue construct that contains large quantities of organ building blocks, which may ultimately be used therapeutically to repair and replace human organs with lab-grown versions containing patients' own cells."
SWIFT 3D bioprinting
Several studies have emerged in recent months demonstrating the potential of 3D bioprinting technology to make way for artificially grown human organs. However, most of the existing strategies for 3D bioprinting result in models with limited cell density and functional capacity, compared with actual adult hearts, restricting their utility for organ repair.
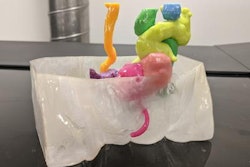
To address these constraints, Skylar-Scott and colleagues developed an alternative method for generating organ tissue. For their technique, they first assembled thousands of stem cells into an organ-like shape. Next, they placed this organ building block into a mold and used centrifugation to generate a living matrix consisting of roughly 200 million patient-specific cells per milliliter.
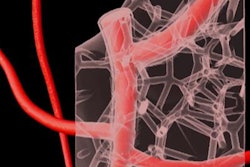
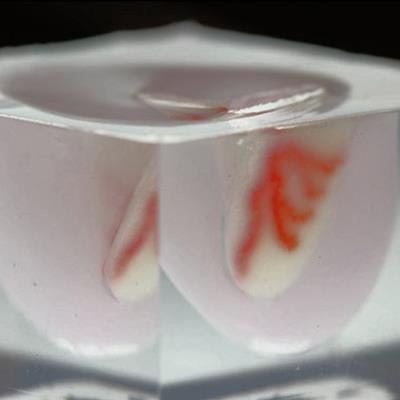
Then they used a 3D printer to inject ink through the tissue matrix. The ink washed away soon thereafter, leaving behind a series of interconnected channels within the tissue that replicated the pattern of a vascular network and allowed for the delivery of oxygen and other nutrients to the tissue. The diameter of the channels varied from 400 µm to 1 mm.
"Forming a dense matrix from these organ building blocks kills two birds with one stone: Not only does it achieve a high cellular density akin to that of human organs, but the matrix's viscosity also enables printing of a pervasive network of perfusable channels within it to mimic the blood vessels that support human organs," co-lead author Sébastien Uzel, PhD, said.
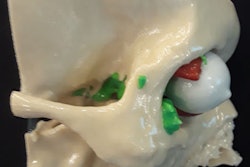
Skylar-Scott and colleagues demonstrated the feasibility of their technique by applying it to the production of a 3D-bioprinted heart model based on the cardiac CT scans of a 17-year-old patient. They obtained the imaging data from the U.S. National Institutes of Health (NIH) 3D Print Exchange database.
After processing the virtual 3D heart model, they 3D bioprinted an arterial vascular network directly into a cardiac building block matrix. They found that the resulting 3D-bioprinted heart model maintained a cell density and microarchitecture that resembled those of actual cardiac tissue. In addition, they pumped a fluid into the model that flowed through the vascular network without issue for several days.
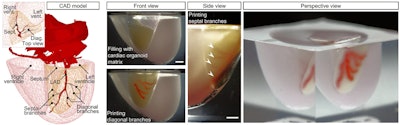

Virtual 3D heart model based on cardiac CT scans of a 17-year-old patient. Left: 3D model of a normal human heart, including a segment of the left anterior descending (LAD) artery and a diagonal branch, from the NIH 3D Print Exchange. Right: A portion of a 3D-bioprinted heart model seen from various angles. Image courtesy of Skylar-Scott et al. Licensed under CC BY-NC 4.0.
'Huge step'
The researchers observed the 3D-bioprinted heart model for a period of seven days, and they found that the organ building blocks began to fuse together to form more solid cardiac tissue and also displayed modest contractility, at about 1% myocardial strain, beating synchronously for the entire week. (Average myocardial strain for adult hearts is about 20%.)
"Our SWIFT biomanufacturing method is highly effective at creating organ-specific tissues at scale from organ building blocks," author Jennifer Lewis, ScD, said. "By integrating recent advances from stem-cell researchers with the bioprinting methods developed by my lab, we believe SWIFT will greatly advance the field of organ engineering around the world."
Skylar-Scott and colleagues are currently collaborating with researchers from Boston University and the Massachusetts Institute of Technology to discover a way to implant the 3D-bioprinted organ tissues into animals requiring organ repair.
"The ability to support living human tissues with vascular channels is a huge step toward the goal of creating functional human organs outside of the body," Wyss Institute founding director Dr. Donald Ingber, PhD, said in a comment on the research. "We continue to be impressed by ... this research, which ultimately has the potential to dramatically improve both organ engineering and the life spans of patients whose own organs are failing."