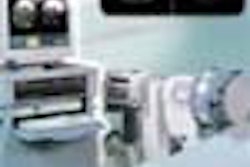
Acist Medical Systems has received Food and Drug Administration 510(k) clearance for its Voyager Model E2000 contrast delivery system. Voyager is designed for use in conjunction with x-ray equipment to diagnose and treat vascular disease, according to Eden Prairie, MN-based Acist.
The Voyager system allows the physician to have instant and variable control over the amount and flow of contrast, enabling him to keep the amount of injected contrast to a minimum, according to Acist.
Voyager provides the physician with one integrated system that handles a variety of injections and remains connected and within the sterile field during the entire diagnostic and/or interventional procedure, according to the firm. It includes an automated syringe operated by Acist's AngioTouch hand controller (a button-operated device), and a touchscreen display that can be used to tailor injections.
In addition, the Voyager System is designed to reduce the amount of contrast media that is wasted at the end of the procedure, employing technology that allows this contrast to be safely used on up to five patients.
Voyager can be used in interve ntional radiological procedures, such as balloon angioplasty, and endovascular surgical procedures such as repair of abdominal aortic aneurysms. Voyager will be launched in early April for the endovascular market. Acist recently showcased Voyager at the 26th annual scientific meeting of the Society of Cardiovascular and Interventional Radiology, held earlier this month in San Antonio, TX.
By AuntMinnie.com staff writersMarch 13, 2001
Related Reading
FDA clears Acist contrast injector, June 5, 2000
Copyright © 2001 AuntMinnie.com