Focused-ultrasound developer InSightec has begun the second stage of a phase I clinical trial for noninvasive treatment of brain tumors using MR-guided focused ultrasound (MRgFUS) surgery on the Haifa, Israel-based company's ExAblate system.
The objectives of the study, approved by the U.S. Food and Drug Administration (FDA), are to evaluate the safety of focused ultrasound delivered through an intact skull and estimate the effect of thermal ablation on tumors.
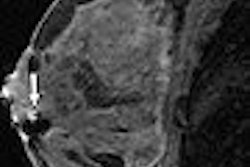
ExAblate uses high-intensity focused ultrasound waves to destroy tissue in combination with MRI. The system also is designed to provide visualization of tumors and acoustic energy beam paths, as well as real-time thermal feedback for physicians to monitor and control the treatment process.
Three patients with recurrent glioblastoma have already been treated at Brigham and Women's Hospital in Boston.
By AuntMinnie.com staff writers
September 26, 2007
Related Reading
InSightec nabs CE Mark, June 7, 2007
InSightec nets Canadian approval, March 12, 2007
InSightec nets FDA clearance for new software, March 5, 2007
FDA clears InSightec's ExAblate 2000 for GE 3T MRI, March 1, 2007
InSightec raises $15 million, August 10, 2006
Copyright © 2007 AuntMinnie.com