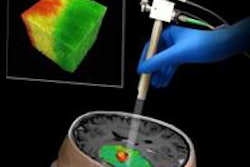
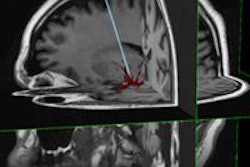
Johns Hopkins University spin-off Clear Guide Medical has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Scenergy ultrasound/CT fusion and image guidance technology.
Designed to help interventional radiologists and surgeons perform minimally invasive biopsies and other diagnostic and therapeutic procedures, Scenergy attaches to most commercially available ultrasound probes and offers an integrated display of fused ultrasound and CT images, according to the company. Scenergy features a fully automated image registration process; CT images are overlaid on the ultrasound plane, Clear Guide Medical said.
Clear Guide noted that Scenergy is not yet available outside of the U.S. market. The company is currently pursuing additional international clearances.