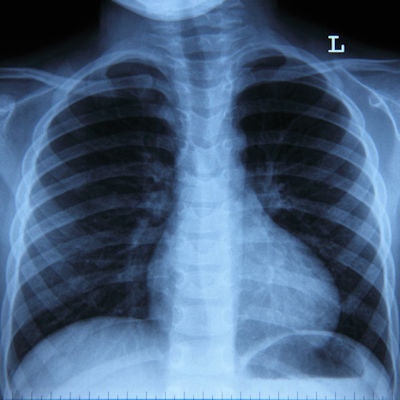
Artificial intelligence (AI) software developer Zebra Medical Vision has received Food and Drug Administration (FDA) 510(k) clearance for HealthCXR to identify and triage pleural effusion on chest x-rays.
The software automatically identifies findings suggestive of pleural effusion based on computed radiography (CR), digital radiography (DR), and dual-energy x-ray absorptiometry (DEXA) scans and notifies radiologists, which allows them to facilitate a quicker diagnosis for the patient, according to the company.
In other Zebra news, the firm is now part of the FDA's Digital Health Software Precertification (Pre-Cert) Program. The pilot program will help develop a future regulatory model that will provide a more streamlined and efficient oversight of software-based medical devices. In the Pre-Cert program, the FDA is proposing that software from precertified companies will continue to meet the same safety and efficacy standards that the agency expects for products that followed the standard path to market.