An artificial intelligence (AI) algorithm that analyzes negative screening mammography exams yielded better predictions for a woman's future risk of breast cancer than risk models based on breast density measurements in a recent study, published online December 17 in Radiology.
After performing retrospective testing on over 2,000 women, a group of researchers from Sweden found that risk scores provided by their deep-learning model correlated better than breast density-derived risk scores with the subject's diagnosis of cancer within a year.
What's more, the "density-based predictors showed decreased performance for more aggressive cancers, whereas the [deep learning]-based predictor did not," wrote a team of authors led by Dr. Karin Dembrower, a breast radiologist and doctoral candidate from the Karolinska Institute in Stockholm.
An important building block
Risk prediction is an important building block of screening programs that are adapted to an individual's risk of breast cancer. Effective risk prediction can improve attendance and confidence in screening programs, according to Dembrower.
High breast density is considered a risk factor for cancer, but current prediction models may not fully exploit all the rich information found in mammography images, according to the researchers. This additional information could potentially identify those women who would benefit from additional screening with MRI.
To see if deep learning could help, the researchers trained and validated an Inception-ResNet-v2 deep neural network using over 11,000 cases diagnosed from women ages 40 to 74 who had received mammograms in the Karolinska University Hospital system between 2008 and 2012. All of the women received screening mammography on full-field digital mammography systems from Hologic.
Risk scores
After analyzing the mammography images, the woman's age at image acquisition, and four acquisition parameters, the deep-learning model output a risk score. The researchers then tested the algorithm on a separate test set of 2,283 women, which included 278 breast cancer cases diagnosed between 2013 and 2014 and within one year of the negative mammogram.
The authors also compared the performance of the algorithm with a risk model based on the age-adjusted percentage of breast density, as well as a risk model based on the age-adjusted dense area of the breast. The density-based measures were calculated using version 10.6 of the publicly available Laboratory for Individualized Breast Radiodensity Assessment (LIBRA) automated breast density estimation software developed by the University of Pennsylvania.
| Performance of AI for predicting future risk of breast cancer | |||
| Risk model based on age-adjusted percentage density | Risk model based on age-adjusted dense area | Age-adjusted deep-learning score | |
| Odds ratio | 1.18 | 1.31 | 1.56 |
| Area under the curve | 0.5 | 0.60 | 0.65 |
| False-negative rate | 39% | 36% | 31% |
The differences between the AI risk scores and the breast density risk models were statistically significant for the area under the curve (p < 0.001), as well as for the false-negative rate (p = 0.006). The difference in the false-negative rate was also more pronounced for more aggressive cancers, according to the researchers.
"The deep neural network overall was better than density-based models," Dembrower said in a statement from the RSNA. "And it did not have the same bias as the density-based model. Its predictive accuracy was not negatively affected by more aggressive cancer subtypes."
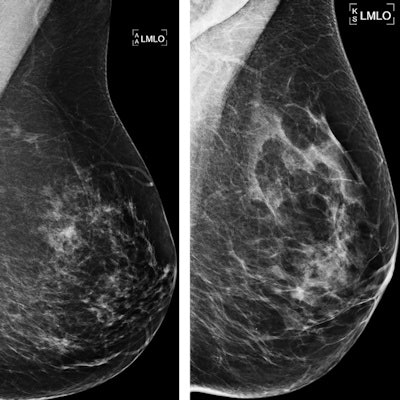
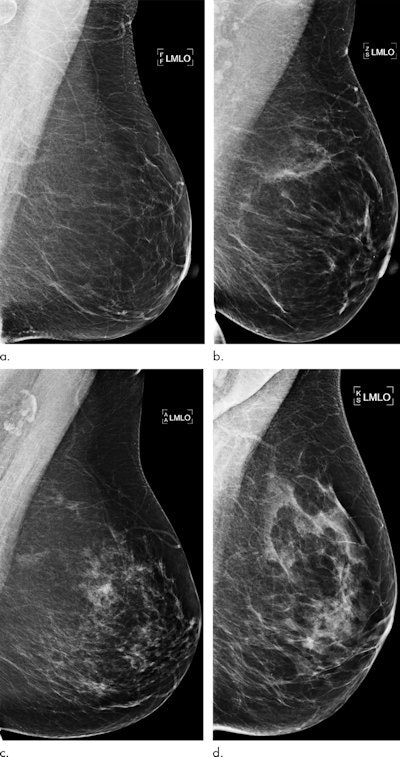 Examples of mammograms with concordance between the deep-learning risk score and the outcome of breast cancer (true predictions). All images are mediolateral oblique views of the left breast. (a) Mammogram in a 55-year-old woman with a low deep-learning risk score (0.05) who was not diagnosed with breast cancer (i.e., true-negative prediction). (b) Mammogram in a 47-year-old woman with a low deep-learning risk score (0.06) who was not diagnosed with breast cancer (i.e., true-negative prediction). (c) Mammogram in a 56-year-old woman with a high deep-learning risk score (0.30) who received a diagnosis of breast cancer 5.1 years after the examination (i.e., true-positive prediction). (d) Mammogram in a 57-year-old woman with a high deep-learning risk score (0.30) who received a diagnosis of breast cancer 5.0 years after the examination (i.e., true-positive prediction). All images courtesy of Radiology.
Examples of mammograms with concordance between the deep-learning risk score and the outcome of breast cancer (true predictions). All images are mediolateral oblique views of the left breast. (a) Mammogram in a 55-year-old woman with a low deep-learning risk score (0.05) who was not diagnosed with breast cancer (i.e., true-negative prediction). (b) Mammogram in a 47-year-old woman with a low deep-learning risk score (0.06) who was not diagnosed with breast cancer (i.e., true-negative prediction). (c) Mammogram in a 56-year-old woman with a high deep-learning risk score (0.30) who received a diagnosis of breast cancer 5.1 years after the examination (i.e., true-positive prediction). (d) Mammogram in a 57-year-old woman with a high deep-learning risk score (0.30) who received a diagnosis of breast cancer 5.0 years after the examination (i.e., true-positive prediction). All images courtesy of Radiology.Direct predictions
Dembrower noted that the institution is not currently reporting mammographic density information.
"In the introduction of individually adapted screening, we use deep learning networks trained to predict cancer rather than taking the indirect route that density offers," she said.
Deep-learning researchers from the Royal Institute of Technology in Stockholm are now working on an update to the model.
"After that, we aim to test the model clinically next year by offering MRI to the women who stand to benefit the most," Dembrower said.