In silico imaging trials represent a viable source of regulatory evidence for imaging device evaluation, according to an analysis conducted by the U.S. Food and Drug Administration (FDA) and published August 9 in Plos Computational Biology.
In the context of medical devices, an in silico clinical trial (ISCT) is a computational study in which a medical intervention is evaluated using a cohort of computational models of patients, wrote lead author Pras Pathmanathan, PhD, a senior FDA research scientist, and colleagues.
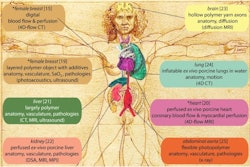
Although an in silico trial is an emerging method for evaluating a medical device that uses computational modeling and simulation (M&S), little information is available on how to ensure reliability with these studies, according to the authors, who highlighted the Virtual Imaging Clinical Trial for Regulatory Evaluation (VICTRE) ISCT project that compared digital breast tomosynthesis (DBT) to full-field digital mammography (FFDM) in one of eight ISCT examples featured.
For that trial, a cohort of 3,000 synthetic breast phantoms was created by sampling a mathematical anatomic model, the investigators noted. Then, FFDM and DBT images were simulated using a physics-based Monte Carlo x-ray transport code. Finally, the detectability of lesions inside the breasts was evaluated using mathematical model observers (i.e., a clinician model).
"The results of the ISCT compared favorably with the results of an existing clinical trial that was used as a reference, indicating that in silico imaging trials represent a viable source of regulatory evidence for imaging devices evaluation," Pathmanathan and colleagues wrote.
ISCTs have many potential applications, including augmenting or reducing the size of real-world clinical trials and supporting trial design by providing improved inclusion-exclusion criteria, the authors explained. Their paper reveals processes and standard approaches to demonstrate the credibility of in silico trial results and considers what verification, validation, and uncertainty quantification mean in the context.
The paper complements the FDA's recent "Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions" guidance published in November 2023 and Model Credibility Regulatory Science Tool.
Read the full paper here.