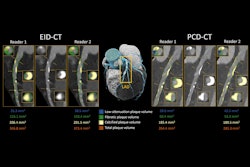
Caristo Diagnostics has received clearance from the U.S. Food and Drug Administration (FDA) for its AI-enabled CaRi-Plaque image-analysis application for coronary plaque detection.
CaRi-Plaque can be used in the noninvasive analysis of coronary anatomy and pathology from routine coronary CT angiography (CCTA) scans to determine the presence, extent, and severity of coronary plaques and luminal stenosis, Caristo explained. The cloud-based software builds on the company's CaRi-Heart application, which is designed to quantify coronary inflammation and characterize plaque on routine CCTA scans and generate a risk score.
CaRi-Heart is currently available for research use only in the U.S.
Caristo notes that when CaRi-Heart receives FDA clearance, clinics that are already using CaRi-Plaque will be able to adopt CaRi-Heart for analysis of both plaque and coronary inflammation.