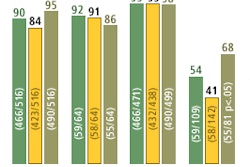
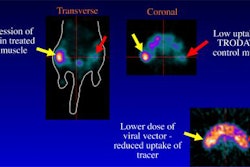
Mirada Solutions has received Food and Drug Administration 510(k) clearance for its Fusion7D multimodality image-fusion workstation software. Fusion7D can support any combination of CT, PET, MRI, and SPECT data, Mirada said. The Oxford, U.K.-based firm is formally introducing Fusion7D at this week's Society of Nuclear Medicine meeting in Los Angeles.
By AuntMinnie.com staff writersJune 19, 2002
Related Reading
Mirada Solutions joins CAD software fray, November 25, 2001
Copyright © 2002 AuntMinnie.com