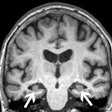
The U.S. National Cancer Institute (NCI) has launched a new initiative under its Small Business Innovation Research (SBIR) program to help small businesses navigate the U.S. Food and Drug Administration (FDA) approval process.
The NCI SBIR Regulatory Assistance Program is designed to help the agency go beyond its traditional mission of providing funding to start-ups. The program offers help in navigating the federal government's regulatory pathway by giving selected companies in the therapeutics and medical device industries up to 30 hours of regulatory consultant time to help them develop comprehensive regulatory plans.
The program is open to companies with phase II grants in the NCI SBIR and Small Business Technology Transfer (STTR) programs. Grants must be current or have ended in the past two years.
Applications will be accepted and undergo a competitive review process until February 4, 2011, with final selection of participants made by March 4, 2011. More information is available at the program's website.
Copyright © 2011 AuntMinnie.com