It's often called the "FUD factor." And nothing causes more fear, uncertainty, and doubt (FUD) in the minds of radiology facility managers than the validation site survey that is required for accreditation under the Medicare Improvements for Patients and Providers Act of 2008 (MIPPA).
MIPPA-mandated site surveys are aimed at ensuring that nonhospital facilities providing advanced diagnostic imaging services (ADIS) continue to meet accreditation standards in the interim between accreditation renewals. But what causes administrators the most anxiety is that MIPPA mandates that the site visits must be unannounced.
What to expect
 Marion Boston, assistant director of validation site survey, ACR.
Marion Boston, assistant director of validation site survey, ACR.During the site validation survey, the surveyor assesses and reports on two areas: deficiencies and recommendations. If a facility has no deficiencies, its accreditation remains in good standing and no further action is necessary. If deficiencies are found, the facility must take corrective action to maintain its accreditation. Corrective action must be submitted within 60 days of the date on report.
Categories of deficiencies include the following:
- Equipment verification
- Personnel documentation
- Physician peer-review program
- Mandatory policies
- Quality assurance and quality control
- Consumer complaint notice posting
- Image labeling
- Patient reports
Unlike deficiencies, recommendations provide nonbinding suggestions for facility improvement.
Survival tips from the ACR
The American College of Radiology (ACR) is responsible for accrediting more than 20,000 facilities in the U.S. As an accrediting body approved by the U.S. Centers for Medicare and Medicaid Services (CMS), the ACR is also responsible for conducting unannounced validation site surveys for nearly 7,000 facilities that provide advanced diagnostic imaging services.
To help ADIS facility managers overcome the FUD factor and proactively prepare for these site surveys, the ACR has compiled and analyzed the most common causes of noncompliance with accreditation requirements. Our goal is to provide facilities with insight into the survey results, specifically highlighting recurring problem areas.
What you need to know
Since 2012, when ACR first began conducting site validation visits under MIPPA, our expert surveyors have conducted unannounced surveys at 4,122 facilities (see table 1). Of the facilities surveyed during that five-year period, 1,943 facilities (47%) were in full compliance with no further action necessary, and 2,179 facilities (53%) had at least one deficiency that required corrective action (see table 2).
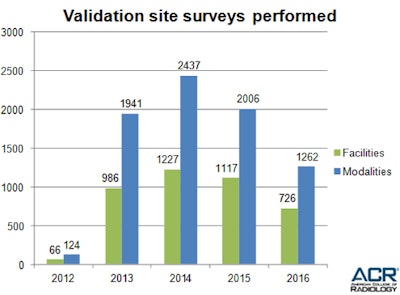 Table 1: ACR performs MIPPA-mandated validation site surveys to ensure that facilities continue to meet accreditation standards between renewals. All tables courtesy of the ACR.
Table 1: ACR performs MIPPA-mandated validation site surveys to ensure that facilities continue to meet accreditation standards between renewals. All tables courtesy of the ACR.During the reporting period, the average number of deficiencies per noncompliant facility was 3.6, and the average number of recommendations per site was 3.2.
Although the site validation process found that a majority of ADIS facilities had deficiencies, no facility has lost accreditation because of an inability to correct the issues identified in the site validation survey.
Most common deficiencies
The prevalence of each type of deficiency is shown in table 3, but here is a breakdown of significant issues:
Policies and procedures (2,486 facilities or 37%): The most common reason for site validation deficiencies was the inability of facilities to provide the surveyor with the eight policies and procedures that are mandated by CMS for accreditation. To see a list of mandatory policies and procedures, follow the link to the ACR Site Survey Toolkit. The facilities reported as deficient either did not have the required policies and procedures or were not able to produce them at the site being surveyed.
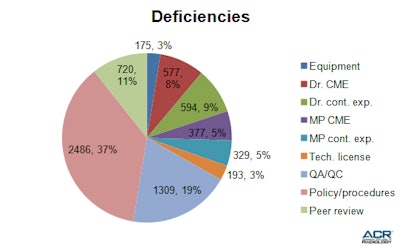 Table 3: The ACR has compiled and analyzed the most common causes of noncompliance with accreditation requirements.
Table 3: The ACR has compiled and analyzed the most common causes of noncompliance with accreditation requirements.A lack of primary source verification for certified personnel was a leading cause of deficient policies, with 744 facilities (26%) failing to provide evidence that they used a primary source to verify the certification of their personnel. Table 4 shows the prevalence of deficient policies among surveyed facilities. It is important to note that a copy of certification is not sufficient to verify credentials. You must contact the primary certification bodies (American Board of Radiology, American Registry of Radiologic Technologists, Nuclear Medicine Technology Certification Board, American Registry of Magnetic Resonance Imaging Technologists, individual state bodies, etc.) for each physician, medical physicist, and technologist on your team.
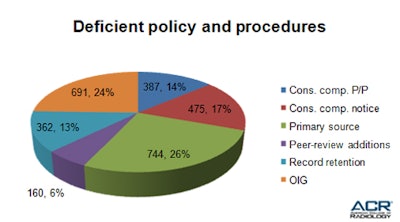 Table 4: Lack of mandatory policies and procedures is the most common reason for site validation deficiencies.
Table 4: Lack of mandatory policies and procedures is the most common reason for site validation deficiencies.Personnel documentation (1,877 or 27%): Coming in second with the next highest number of reported deficiencies was the lack of proper personnel documentation for radiologists, physicists, and/or technologists. A significant number of sites were unable to produce records of continuing medical education or continuing experience for their personnel.
Quality assurance and quality control (1,309 facilities or 19%): The third highest number of deficiencies reported across all facilities was the lack of proper paperwork to document quality assurance and quality control measures.
Nonaccredited equipment (175 facilities or 3%): While this category of deficiency was reported for the smallest number of facilities, it is one of the most critical failures within the site validation process. All modalities, including all modules, and units being used for ADIS must be submitted for accreditation. If you get new equipment in the three-year interim between accreditation, you must let the ACR know and apply for accreditation for that modality or unit.
Most common recommendations
Most facilities undergoing a site validation survey find that the recommendations for improvement are one of the most valuable outcomes of the process.
In the analysis, recommendations for MR safety (458 facilities or 25%) topped the list of those most frequently cited, closely followed by suggestions for improved communication (360 facilities or 19%).
Table 5 shows the most common recommendations made in reports to ADIS facilities.
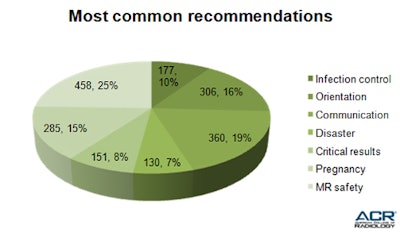 Table 5: Recommendations for improvement are one of the most valuable outcomes of the site validation survey process.
Table 5: Recommendations for improvement are one of the most valuable outcomes of the site validation survey process.Tools to help you succeed
The ACR provides critical tools and expertise to help facilities with advanced diagnostic imaging services be better prepared for validation site surveys, reducing the likelihood of facility noncompliance. Here are links to some of the resources you will find invaluable to enhance the success of your site validation survey.
ACR Site Survey Toolkit: One of the most valuable resources is the ACR Site Survey Toolkit, which provides a complete checklist to help you gather and maintain the documentation that is required for accreditation and that will be requested during the survey.
Consumer complaint notice: ADIS facilities must post (in their waiting rooms) a notice of the process consumers can use to file a complaint. Here is a standard consumer complaint notice that facilities can download.
Primary source verification: Here is the link to conduct primary source verification for board-certified physicians and medical physicists. Here is the link to verify that technologists are ARRT-certified.
Fraud protection: To search for proof of personnel fraud, search the Office of Inspector General Exclusion list here.
Marion Boston is the assistant director of validation site surveys with the American College of Radiology.
The comments and observations expressed herein are those of the author and do not necessarily reflect the opinions of AuntMinnie.com.