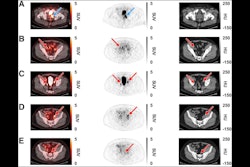
BAMF Health has launched a nationwide radiopharmaceutical-focused clinical trials network to support research, development, and commercialization.
Called BAMF RadioNexus, the platform is designed to connect health systems, academic medical centers, and independent practices, as well as provide expertise for radiopharmaceutical trial protocol design, regulatory navigation, and patient access, according to BAMF.
"As innovation in the field accelerates, there’s a growing need for highly qualified sites equipped with the specialized infrastructure, expertise, and support required to run these complex trials," BAMF Health said in its announcement.
Logistics and infrastructure required to initiate and complete radiopharmaceutical clinical trials are complex, BAMF noted. BAMF RadioNexus enables accelerated study startup and site activation, while offering access to a growing network of diverse patient populations. Platform partners also have priority access to novel diagnostic and treatment trials.
The nationwide platform expands patient access to radiopharmaceutical clinical trials by enabling more sites across the country, BAMF said, adding that one national health system is already enrolled.