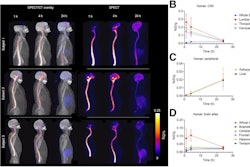
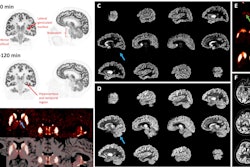
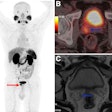
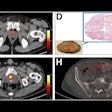
Nuclear medicine appears ahead of the curve when it comes to replacing animal testing with more effective methods that will accelerate the evaluation process and lower R&D costs for new drugs.
In a recent announcement, the U.S. Food and Drug Administration (FDA) said it is taking a “groundbreaking step” by reducing animal testing in preclinical safety studies with scientifically validated new approaches, and offered a strategic roadmap.
Nuclear medicine experts have been involved in the initiative since August 2023, when a group was invited to Washington, DC, to give advice on what types of studies could be cut. First on the list was dosing safety studies for new PET radiotracers, which currently follow the same development pathway as pharmaceuticals, said Peter Scott, PhD, of the University of Michigan (UMich) in Ann Arbor.
“They ultimately came to the conclusion that there was enough information in the literature to justify eliminating the need for preclinical dosimetry for PET drugs labeled with short-lived radionuclides,” he said.
Scott, director of nuclear medicine and molecular imaging at UMich, and colleagues outlined the new approach in an article in the June issue of the Journal of Nuclear Medicine. Essentially, sufficient data are available to justify omitting preclinical dosimetry studies for new radiopharmaceuticals labeled with F-18, carbon-11, gallium-68, copper-64, rubidium-82, and nitrogen-13, they explained.
“Several of us have recently used this new approach, filing an IND application for a [first-in-human] study with a Cu-64 labeled radiopharmaceutical without a preclinical dosimetry package, and were gratified that the FDA is following this approach and approved the study to proceed,” the group wrote.
Ultimately, the cost saving could be huge, but more importantly, eliminating dosimetry animal studies will speed up patient access to new PET agents, Scott noted.
Next up in terms of what may be eliminated could be pharmacology and toxicology (“pharm-tox”) animal studies, which are also currently required by the FDA for new PET imaging agents, Scott added.