GE HealthCare (GEHC) has debuted its Signa Sprint ultrapremium wide bore 1.5-tesla high-performance gradient MRI system at the International Society for Magnetic Resonance in Medicine (ISMRM) meeting in 2025.
Pending 510(k) clearance from the U.S. Food and Drug Administration (FDA), Signa Sprint is designed for advanced imaging capabilities in cardiology, oncology, and other clinical and research areas. The product will equip clinicians and researchers with the ability to expand current 1.5-tesla boundaries while improving the patient experience, according to GEHC.
 GE HealthCare debuted its Signa Sprint ultrapremium wide bore 1.5-tesla high-performance gradient MRI system at the International Society for Magnetic Resonance in Medicine (ISMRM) 2025 meeting.GE HealthCare
GE HealthCare debuted its Signa Sprint ultrapremium wide bore 1.5-tesla high-performance gradient MRI system at the International Society for Magnetic Resonance in Medicine (ISMRM) 2025 meeting.GE HealthCare
"Signa Sprint is designed to offer high-performance scanning exceptional diffusion imaging, a critical tool in oncology diagnosis and treatment planning," stated GEHC in its related announcement.
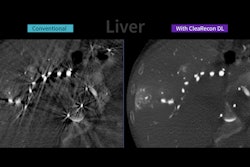
Described as a next-generation, high-performance platform, Signa Sprint features built-in AI technologies AIR Recon DL, Sonic DL, and AIR x, according to GEHC. The system aims to enable accelerated cardiac MRI through the use of deep-learning reconstruction techniques to help reduce the time and expertise needed to interpret scans and drive consistency and reliability, the company added.
At ISMRM 2025, GEHC also showcased the following:
- Signa Magnus, a head-only MR scanner designed to support high resolution, high signal-to-noise ratio, diffusion-weighted imaging, and short scan times. This scanner features the HyperG gradient coil.
- Sonic DL 3D, which is designed to accelerate MRI scans across a wide range of clinical applications.
- Freelium, GE's helium-free sealed magnet platform in development that aims to dramatically reduce liquid helium usage.