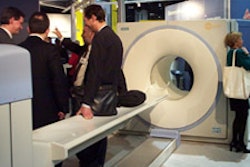
CHICAGO – Dutch x-ray firm Nucletron this week at RSNA introduced ThoraScan, a new digital chest system. The work-in-progress ThoraScan employs the Veenendaal, Netherlands firm’s slot scanning technology in concert with a charge-coupled device digital detector provided by Thomson Tubes Electronique.
ThoraScan employs a 1-cm thick, fan-shaped x-ray beam -- an image acquisition technique that reduces scatter radiation and offers 40% dose reduction compared with traditional systems, according to the company. The system is the digital version of Oldelft’s Amber chest unit, and is the result of a combined effort between Nucletron and Oldelft’s Diagnostic X-ray group, which Nucletron acquired in 1999.
The Thomson CCD/Time Delay Integration detector uses a cesium iodide tallium-doped scintillator. The x-ray signal is digitally processed inside the detector for direct transmission to workstations or to a network in DICOM 3.0 format. Thorax scanning time is one second, and the total response time from x-ray exposure to the availability of the image on the operator workstation is four to five seconds, according to the vendor.
Nucletron believes a key distinguishing feature of ThoraScan is its “pre-processing” of the x-ray signal. The system is able to change and modulate the primary x-ray beam based on a preliminary “light” scan of the upper anatomy of the thorax, said Julius Fortuna, product specialist.
“The system automatically determines the proper milliamperes (mA) in the generator, and then when it starts the scanning process, it can change and manipulate the primary x-ray beam to (fit) the exact dose requirements of that patient,” he said.
ThoraScan’s standard resolution mode will deliver a resolution of 162 microns, with a modulation transfer function (MTF) at 15%, 3.2 line pairs/mm, and detective quantum efficiency (DQE) of 60%. In addition, ThoraScan will also be equipped with a high-resolution mode, allowing for resolution of 81 microns with a MTF at 5% and 5 line pairs/mm -- similar to digital mammography images, according to Raymond Horn, director of sales support.
Also included on ThoraScan is Agfa’s Musica post-processing algorithm. ThoraScan is currently pending U.S. Food and Drug Administration 510(k) clearance, and will be available to customers in the fourth quarter of 2001, according to the company. Beta testing of ThoraScan will take place at the Duke University Medical Center in Durham, NC, and the University of California, San Diego Medical Center.
ThoraScan will have a list price of approximately $300,000, Fortuna said. It is also available with PACS equipment contributed by fellow Dutch firm Rogan Medical Systems.
By Erik L. Ridley
AuntMinnie.com staff writer
November 27, 2000
ThoraScan employs a 1-cm thick, fan-shaped x-ray beam -- an image acquisition technique that reduces scatter radiation and offers 40% dose reduction compared with traditional systems, according to the company. The system is the digital version of Oldelft’s Amber chest unit, and is the result of a combined effort between Nucletron and Oldelft’s Diagnostic X-ray group, which Nucletron acquired in 1999.
The Thomson CCD/Time Delay Integration detector uses a cesium iodide tallium-doped scintillator. The x-ray signal is digitally processed inside the detector for direct transmission to workstations or to a network in DICOM 3.0 format. Thorax scanning time is one second, and the total response time from x-ray exposure to the availability of the image on the operator workstation is four to five seconds, according to the vendor.
Nucletron believes a key distinguishing feature of ThoraScan is its “pre-processing” of the x-ray signal. The system is able to change and modulate the primary x-ray beam based on a preliminary “light” scan of the upper anatomy of the thorax, said Julius Fortuna, product specialist.
“The system automatically determines the proper milliamperes (mA) in the generator, and then when it starts the scanning process, it can change and manipulate the primary x-ray beam to (fit) the exact dose requirements of that patient,” he said.
ThoraScan’s standard resolution mode will deliver a resolution of 162 microns, with a modulation transfer function (MTF) at 15%, 3.2 line pairs/mm, and detective quantum efficiency (DQE) of 60%. In addition, ThoraScan will also be equipped with a high-resolution mode, allowing for resolution of 81 microns with a MTF at 5% and 5 line pairs/mm -- similar to digital mammography images, according to Raymond Horn, director of sales support.
Also included on ThoraScan is Agfa’s Musica post-processing algorithm. ThoraScan is currently pending U.S. Food and Drug Administration 510(k) clearance, and will be available to customers in the fourth quarter of 2001, according to the company. Beta testing of ThoraScan will take place at the Duke University Medical Center in Durham, NC, and the University of California, San Diego Medical Center.
ThoraScan will have a list price of approximately $300,000, Fortuna said. It is also available with PACS equipment contributed by fellow Dutch firm Rogan Medical Systems.
By Erik L. Ridley
AuntMinnie.com staff writer
November 27, 2000
Copyright © 2000 AuntMinnie.com
Click here to view the rest of AuntMinnie’s coverage of the 2000 RSNA conference.
Click here to post your comments about this story.