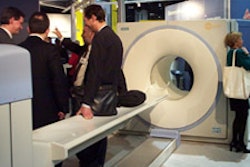
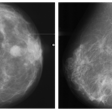
CHICAGO - Mammography developer Fischer Imaging is laying the groundwork for the commercial launch of its SenoScan full-field digital mammography system. The Denver company yesterday announced a technology collaboration with computer-aided diagnosis firm CADx Medical Systems of Laval, Quebec.
The companies have agreed to integrate their respective technologies, with the goal of enabling SenoScan to send images seamlessly to CADx’s Second Look CAD system. Second Look reviews digitized mammograms and highlights suspicious areas that might require further attention.
The agreement will strengthen the competitive positions of each company when their products reach the U.S. market by enabling them to offer additional functionality to customers. In a press conference on Tuesday, Fischer chairman and CEO Morgan Nields highlighted the ability of Second Look to find cancers that might have been missed by radiologists.
The deal is nonexclusive, and each company is free to work with other vendors. The firms have not yet determined whether they will market SenoScan and Second Look as part of a bundled package.
Fischer is putting the final touches on the company’s FDA application for SenoScan, according to Nields. Fischer’s efforts have been delayed by changing requirements at the FDA regarding regulation of full-field digital mammography, but the company should be ready in the next several months to submit either a 510(k) or a premarket approval (PMA) application with data from the multicenter study it has conducted with SenoScan.
Fischer is already taking orders from European customers, and expects to begin shipping to Europe in the first quarter of 2001, Nields said. European regulatory requirements regarding full-field digital mammography are less rigorous than those in the U.S., and the company will be able to begin shipping systems after self-certifying the product under the CE Mark process.
CADx is also moving toward the final phase of the regulatory process for Second Look in the U.S, according to vice president of commercial operations Jim Corbett. The company has submitted two of three modules for its PMA, and expects to submit the third module by the end of the year, Corbett said. The hard-copy version of the system has already been launched in international markets.
By Brian Casey
AuntMinnie.com staff writer
November 29, 2000
The companies have agreed to integrate their respective technologies, with the goal of enabling SenoScan to send images seamlessly to CADx’s Second Look CAD system. Second Look reviews digitized mammograms and highlights suspicious areas that might require further attention.
The agreement will strengthen the competitive positions of each company when their products reach the U.S. market by enabling them to offer additional functionality to customers. In a press conference on Tuesday, Fischer chairman and CEO Morgan Nields highlighted the ability of Second Look to find cancers that might have been missed by radiologists.
The deal is nonexclusive, and each company is free to work with other vendors. The firms have not yet determined whether they will market SenoScan and Second Look as part of a bundled package.
Fischer is putting the final touches on the company’s FDA application for SenoScan, according to Nields. Fischer’s efforts have been delayed by changing requirements at the FDA regarding regulation of full-field digital mammography, but the company should be ready in the next several months to submit either a 510(k) or a premarket approval (PMA) application with data from the multicenter study it has conducted with SenoScan.
Fischer is already taking orders from European customers, and expects to begin shipping to Europe in the first quarter of 2001, Nields said. European regulatory requirements regarding full-field digital mammography are less rigorous than those in the U.S., and the company will be able to begin shipping systems after self-certifying the product under the CE Mark process.
CADx is also moving toward the final phase of the regulatory process for Second Look in the U.S, according to vice president of commercial operations Jim Corbett. The company has submitted two of three modules for its PMA, and expects to submit the third module by the end of the year, Corbett said. The hard-copy version of the system has already been launched in international markets.
By Brian Casey
AuntMinnie.com staff writer
November 29, 2000
Copyright © 2000 AuntMinnie.com
Click here to view the rest of AuntMinnie’s coverage of the 2000 RSNA conference.
Click here to post your comments about this story.