Alpha-emitting astatine-211 (At-211) may open the door to new targeted radioimmunotherapy for treating blood cancer, according to research presented June 10 at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) in Toronto.
Yawen Li, PhD, from the University of Washington (UW) Molecular Radiotherapy Laboratory in Seattle, shared what researchers have learned about production, labeling, and potential application of astatine, an element of interest due to its long half-life of 7.21 hours.
"[At-211 lasts] long enough for logistic feasibility in the clinic," Li said during her talk, "yet [clears quickly] enough to allow transplant without residual radioactivity in the blood."
Other advantages of At-211 include the following:
- It emits one high-energy alpha particle per decay that deposits a larger dose of radiation within a few cell diameters.
- It does not have alpha-emitting daughter(s) from its decay.
- It responds to available antibody labeling methods, and the labeled antibodies have adequate in vivo stability to be able to control nontargeted toxicity.
- It can be produced by irradiating a naturally abundant bismuth target using a moderate-energy alpha beam. Bismuth target material is both inexpensive and readily available.
- There is currently an adequate supply of At-211 to conduct dose escalation studies in clinical trials and to be extended to multisite clinical trials.
The work could lead to targeted alpha therapy as an alternative to total-body irradiation, according to Li.
"Our long-term goal is to replace total body irradiation and conditioning regiments with alpha radioimmunotherapy," she said. "We believe alpha radioimmunotherapy can reduce toxicity of conditioning regiment for hematopoietic cell transplantation and improve disease control which will ultimately extend this treatment to nonmalignant diseases, medical infirmed patients, and pediatric patients."
Alpha radioimmunotherapy targets subsets of cells in the immune system, increases specific immunosuppression, and results in less toxicity to nontarget tissues than total body irradiation, Li noted.
The Molecular Radiotherapy Lab team's work uses a Scanditronix MC-50 medical cyclotron equipped with multiple beam lines and capable of producing protons, neutrons, and alpha beams at amperages useful for radionuclide and At-211 production. Li and colleagues are currently using one beam line for fast neutron therapy, one for proton therapy research, and two lines for radioisotope production; an additional beam line is under construction.
At-211 production involves a wet chemistry liquid-liquid extraction method for isolating astatine from irradiated bismuth targets. Production starts by dissolving the target in concentrated nitric acid, Li explained, then involves removing the nitric acid by distillation, dissolving the residue in hydrochloric acid, and performing multiple liquid-liquid extraction steps using diisopropyl ether, with the astatine extracted into sodium hydroxide, followed a pH adjustment for antibody labeling.
The process has consistently provided 60% isolation yields for antibody labeling, Li said, but it requires a highly skilled radiochemist to perform the procedure. Thus, to reduce dependence on highly skilled personnel -- and to minimize radiation exposure to the team -- the group developed a semiautomated astatine isolation method that uses a tellurium metal powder to extract astatine from a hydrochloric acid solution and then recovers the astatine using a solution of sodium hydroxide.
The overall runtime for the process is about one hour, and the current protocol provides an average isolation yield of about 80%, Li added.
As for At-211 labeling of antibodies, Li mentioned notable differences between astatine and iodine.
Astatine cannot be stably attached to tyrosine residues of antibodies, so an intermediate bifunctional reagent containing a functional group for antibody conjugation is required for antibody astatination. N-succinimidyl ester, isothiocyanate, or maleimide groups are commonly used for this purpose, according to Li, who added that the most common astatine labeling strategy involves aryl-trialkylstannane derivatives.
Preclinical research at UW involves collaborating with different investigators at Fred Hutchinson Cancer Center. Projects span multiple directions, including targeting latent HIV-infected cells; alpha RIT for lymphoma and leukemias (targeting CD20, CD45, CD33, CD123, and CD117); alpha RIT for multiple myeloma (targeting CD38); RIT to study graft-vs-host disease; and combining targeted RIT and synergistic novel agents to eradicate acute myeloid leukemia.
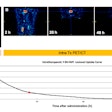
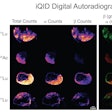
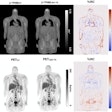
Li brought results from two preclinical multiple myeloma studies of At-211-CD38 therapy in male and female mice bearing disseminated OPM-2 Luc disease. Both studies confirmed that long-term survival can be provided for the majority of the mice at dose levels (45 µCi, 30 µCi, and 15 µCi) that do not cause significant kidney or liver toxicity, Li said. The studies support further clinical investigation.
 Preclinical multiple myeloma studies at the University of Washington, presented during SNMMI 2024, show survival of mice at different intervals and doses of the alpha radioimmunotherapy At-211-CD38. Graphic and caption courtesy of Yawen Li, PhD.
Preclinical multiple myeloma studies at the University of Washington, presented during SNMMI 2024, show survival of mice at different intervals and doses of the alpha radioimmunotherapy At-211-CD38. Graphic and caption courtesy of Yawen Li, PhD.
The UW team is also evaluating CD45 antibody in three phase I and II clinical trials that were on hold at the time of the SNMMI annual meeting, due to U.S. Food and Drug Administration (FDA) requirements that included a need for imaging studies in dosimetry. Those studies will resume once all FDA requirements are met. Additionally, a phase I clinical trial focused on CD38 has been approved by the FDA, and patient recruitment will begin in November, Li said.
Click here for our full SNMMI coverage.