Renal Collecting System (Ureteropelvic junction) Obstruction
Although some use the terms hydronephrosis and hydroureter to describe dilatation of the collecting system secondary to obstruction, and the terms caliectasis and pelviectasis denote dilatation, but not necessarily obstruction, others use the term hydronephrosis to describe non-specific collecting system dilation. Causes of hydronephrosis and hydroureteronephrosis include
- Obstruction
- Previous obstruction
- Vesicoureteral Reflux
- Recent urinary tract infection
- Congenital malformations
- Noncompliant bladder
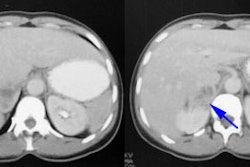
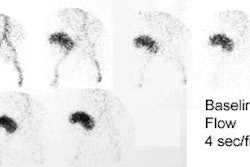
Asymptomatic hydronephrosis without obstruction is not associated with increased morbidity. When the obstruction is complete, however, the renal parenchyma atrophies and renal size decreases (obstructive nephropathy). In partial obstruction, the degree of parenchymal thinning is directly proportional to the severity of obstruction. Glomerular function (evaluated with Tc-DTPA) is more sensitive to ureteral obstruction than is tubular secretion (evaluated with MAG3), although once tubular changes occur, they take longer to reverse.
Prenatal hydronephrosis (collecting system dilatation) can be
identified on prenatal ultrasound in about 1% of patients
(0.3-4.5% [9]) [6] and is bilateral in 37-57% of cases [9].
Followup is generally recommended if the AP diameter of the
collecting system is 7-10 mm during the 3rd trimester of pregnancy
[9]. The first follow up exam should be performed during the 1st
week after birth- but not during the first 72 hours because of
reduced urine output after delivery [9]. On post-natal follow-up,
the dilatation will resolve in 20-50% of cases [6]. The most
common cause of persistent hydronephrosis is ureteropelvic
junction obstruction (UPJ - seen in approximately one in every
20,000 live births [12]), while vescioureteral reflux accounts for
9-15% of cases [9](although some authors report that less than 15%
of cases will be related to a ureteropelvic junction obstruction
[6]). UPJ has a left sided predominance, can be bilateral in
16-25% of cases and is more frequent in males (2-4:1) [9,16].
The goal of post-natal management of asymptomatic unilateral hydronephrosis is to identify patients with severe urinary flow restriction and compromised renal function so that decisions can be made about further treatment [6]. Although there is general agreement that diuretic renography is accurate in adults, recent opinion is changing in neonates and infants with congenital unilateral ureteropelvic junction obstruction [9]. The recent trend in infants is toward less aggressive/conservative management of the dilated asymptomatic collecting system, even when unilateral poor function or obstructive diuretic renography findings are present on initial scanning [1]. Careful followup can be performed safely as the natural history of dilation seems to be resolution or stability of dilation and renal function in this group (only 20-25% if infants managed conservatively require surgery during the first 2 years of life [9]). However, others prefer to operate before there is loss of function and avoid repetitive invasive imaging studies [7].
The Whitaker test (pressure perfusion study) has been considered
the gold standard for distinguishing obstructive from
non-obstructive hydronephrosis. However, this status has been
questioned because of non-physiologic flow rates, lack of
pediatric normal limits, presence of an indeterminate range in a
purported gold standard, and lack of studies correlating its
results with deteriorating function. The exam measures the
pressure within the renal pelvis under conditions of increased
flow. A pressure greater than 22 cm H2O at a flow rate
of 10 mL/min. is felt to be representative of obstruction (normal
is less than 15 cm H2O). Unfortunately, the examination
is invasive (a percutaneous nephrostomy is required) and fails to
provide any information regarding renal function. At the present,
many believe that a prospective imaging gold standard does not
exist for chronic obstruction, and that the diagnosis is made
retrospectively by demonstrating loss of function or relief of
symptoms with an intervention.
Several causes have been postulated as the etiology for UPJ
obstruction including intrinsic and extrinsic causes [16].
Extrinsic etiologies include a crossing vessel (implicated in
11-79% of cases of UPJ- the lower pole segmental artery and vein
in particular), periureteral fibrosis, or abnormal renal rotation
[12,16]. Intrinsic causes include an aperistaltic ureteral
segment, an intrinsic luminal narrowing, and an insertional
abnormality [12,16].
Older patients can present with flank pain, hematuria, renal
stones, and UTI/pyelonephritis [12]. The pain may be intermittent
and may be related to large fluid intake or fluids associated with
a diuretic effect [12].Dietl's crisis (also known as
"beer-drinker's hydronephrosis) refers to a UPJ obstruction that
manifests as intermittent flank pain that typically occurs after a
a large volume of fluid or beer intake [14].
Furosemide Renography
Technique
Non-diuretic renography is extremely sensitive but very non-specific for obstruction. Furosemide renography is based on the principle that prolonged retention of tracer in a dilated, non-obstructed collecting system is due to a reservoir effect and that diuresis will cause increased urine flow through the collecting system which should "wash out" the activity. In the presence of significant obstruction, the urinary tract is unable to clear and activity will not change significantly.
The examination can be performed on neonates less than one month old [4], but it is usually recommended to delay the exam until the age of 4 weeks as the immature renal tubules may not respond to lasix [9]. For infants and young children, prior to the exam oral hydration should be offered as formula or water beginning 2 hours before the study and throughout the exam [4]. IV fluid can be given as a dilute saline solution (D5 0.3 NS or D5 0.25 NS) at a rate to deliver 15 mL/kg over a 30 minute interval, beginning 15 minutes before injecting the radiopharmaceutical [4]. The infusion is continued for the remainder of the study at a maintenance fluid volume at a rate of 200 cc/kg/24 hours [4]. Adults can be given oral hydration prior to the exam.
Tc-MAG3 is the agent used for the examination at a dose of 1.85
MBq/kg (50uCi/kg) and a minimum dosage of 37MBq (1mCi). The adult
dose is 10 mCi. The patient is supine during the exam, but seated
imaging may provide superior information which emphasizes the
importance of gravity assisted drainage [13]. Digital data (at 20
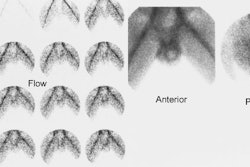
sec/frame) and analog images are acquired [4].
The standard dose of lasix (furosemide) is 0.5 mg/kg up to 40 mg
in an adult and 1.0 mg/kg with a maximum dose of 20 mg in a child
[13]. In young adults with normal renal function a 40 mg dose of
lasix produces a flow rate of approximately 20 mL/min within 3-6
minutes after administration, although maximum diuresis may not be
achieved until 15-18 minutes after injection [13]. When there is
impaired renal function, a larger dose of lasix is required to
attain an effective level of diuresis [13]. Some authors suggest
that the lasix dose needs to be doubled if the Tc-MAG3 clearance
is decreased by half [13].
Furosemide is a sulfonamide-containing agent and is
contraindicated in patients with sulfa allergies. In patient with
sulfa allergies or if lasix is not available, bumetanide is an
acceptable alternative (the agent also acts at the level of the
renal tubules) [13]. The dose of bumetanide is approximately
1/40th the dose of lasix to achieve an equivalent diuretic
response during the first 30 minutes after diuretic administration
[13]. Ethacrynic acid is another alternative agent [15]. However,
one author suggests that lasix can be administered to patients
with sulfa allergies with a very low risk for minor reactions
[15].
A variety of protocols are used for timing of the lasix injection
including F-15 (15 minutes prior to tracer administration), F = 0,
F +2, F + 5, F + 10, and F + 20 [8,13]. The F-15 protocol is
reported to allow better discrimination between obstruction and
normal kidneys, but both the F-15 and F = 0 protocols may simply
demonstrate rising parenchymal activity without visualization of
tracer in the collecting system (a pattern that may fail to
distinguish between reduced renal function and obstruction) [13].
We prefer to perform a baseline renal exam for 20 or 30 minutes
followed by a separate post lasix exam acquisition. A normal
baseline exam will remove the need for a lasix study and to allow
activity to clear from the renal parenchyma. At the end of the
standard exam a non-catheterized patient should stand up, go to
the toilet, and void. A post void image with the patient in the
supine position should then be obtained. This has two purposes-
the collecting system may clear (in which case a lasix exam is not
required) and it also empties the bladder (a distended ballder can
slow collecting system drainage). If collecting system activity
persists, then a lasix examination is performed. We inject lasix
IV at a dose of 1mg/kg (maximum 20 mg in children and 40 mg in
adults [9]) at the start of the diuretic portion of the study
(when the entire collecting system is believed to be full). Images
are acquired for 30 minutes following lasix administration.
Following the lasix exam, if a large amount of activity remains in
the collecting system gravity assisted post void images should be
obtained (this is particular important in pediatric patients)
[13]. The patient should be asked to get off the table, stand up,
walk around for a few minutes, then return to the table for a post
void image [13], Infants should be held in an upright position for
5 minutes to allow gravity to assist drainage [13].
Although the practice is not universal, some centers routinely
catheterize patients for the exam to ensure adequate drainage
throughout the study [4]. Catheterization is particularly useful
in adults or children who cannot empty their bladder.
Catheterization may also be required for children with grade II
vesicoureteral reflux or higher as reflux can cause "saw-tooth"
waves on the down-sloping portion of the renogram curve that can
affect T1/2 determination [4,5].Patients with urinary diversion
procedures (ileal conduit urinary diversion, Indiana pouch
reservoir, and neobladder construction with drainage to the
urethra) may all be predisposed to reflux (although reflux is most
common with an ileal conduit) [13]. When feasible, a urinary
diversion should be catheterized before the study to empty any
residual urine and the catheter should be kept in place during the
study to allow drainage and minimize the possibility that reflux
will compromise exam interpretation [13].
A wide clinical variance in the performance furosemide renography has led to the publication of three consensus statements which are very similar in their methods and interpretive criteria:
- SNM Nuclear Medicine Procedure Guidelines or Pediatric Diuretic Renography
- The "Well Tempered Diureitic Renogram" [2]
- Consensus statement from the 9th International Radionuclides in Nephrourology Meeting, Committee on Diuretic Renography [3]. This statement had the most detailed recommendations for interpretation.
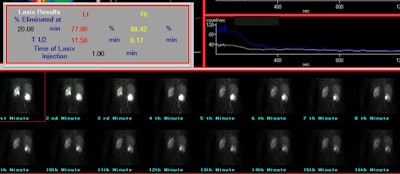
Following the lasix exam, a ROI should be drawn around the dilated collecting system and a curve generated of the rate of clearance of activity from the collecting system. Data indicate that placement of the ROI around the dilated pelvis and collecting system (rather than the whole kidney) better assess the lasix response [13]. A collecting system without significant obstruction will have a clearance half-time (ie: the time for half of the activity present to clear) following lasix administration of less than 10 minutes. Greater than 20 minutes is abnormal and is associated with high grade obstruction. Clearance half-times between 10 and 20 minutes are considered indeterminate, but the patient should be followed closely. The T1/2 is best measured from the point of response to furosemide (not necessarily from the point at which furosemide is given). The response to furosemide usually begins 1 to 2 minutes after injection, but maximum diuresis may not be reached until 15 minutes in some patients. It is important to document that the patient did have a good diuretic response to lasix as dehydration or poor renal function can affect collecting system clearance. Adults should produce 300-400 ml of urine at the end of 30 minutes. A post void volume should be recorded after completion of the lasix exam.
There is generalized agreement that prompt clearance of tracer
from the renal collecting system after lasix with a T1/2 under 10
minutes excludes obstruction [13]. However, a prolonged T1/2
should not be the sole criterion upon which a determination of
obstruction is based [13]. Its value should be interpreted
in conjunction with the other data available from the exam- curve
analysis, differential function, and visual analysis of the
acquired images. The washout curve from a non-obstructed kidney is
more likely to have an exponential or concave shape, even if
washout is prolonged. A convex shape is more indicative of
obstruction. A biphasic curve (one which drops quickly, but then
levels off at a high level or even rises) is almost always
indicative of a flow dependent obstruction- particularly when the
curve levels off above 30% of retained activity. Obstruction
usually causes a loss of renal function - a differential renal
function below 40% or a decrease of differential renal function of
more than 5% on successive diuretic exams is considered indicative
of renal function deterioration and is often used as the threshold
for surgery [9]. Obstruction will also result in delayed tissue
transit from the parenchyma into the collecting system [13]. In a
patient with suspected unilateral obstruction, the likelihood for
obstruction is reduced when the relative renal function is
unchanged from prior exams even if the T1/2 is abnormal [13].
Improvement in the washout of the tracer after pyeloplasty varies greatly. In many cases, the half-time remains in the obstructed or equivocal range for at least 6 months. If function remains stable during this interval, however, there is no need for concern.
|
Dilated non-obstructed right collecting system: The right collecting system demonstrates prompt washout of tracer with a T1/2 of 6.2 minutes. The washout curve is somewhat convex in shape, but there is only about 11.5% retained collecting system activity and the findings were most consistent with a dilated non-obstructed system. |
|
|
Factors which affect half-time clearance include:
Despite optimum technique between 10-15% of studies will be difficult to interpret because of an intermediate diuretic response [13].1- Urine flow rate response to the diuretic:
- Patient hydration status: Dehydrated patients will have less of a response to lasix and this can result in false positive or indeterminate findings [11,13]. Therefore, adequate hydration is essential for diuresis. Adults should consume 300-500 ml of water prior to the exam. In infants and children, 5% dextrose in one-third normal saline (15 mL/kg) is infused over 30 minutes prior to the exam (WTDR). A patient with normal renal function should produce about 200-300 mL or urine in 20-30 minutes after 40 mg of lasix [13].
- Diuretic dose: 1mg/kg up to 40 mg has been shown to be optimal. In very obese patients or in patients with impaired renal function, doses larger than 40 have been proposed, although the Radionuclides in Nephrology consensus statement points out that there is no data to support increased furosemide doses in azotemic patients. Maximum diuresis usually occurs within a few minutes after furosemide injection in the hydrated patient.
- Renal immaturity: In neonates, renal immaturity may produce false positive results [7].
- Degree of renal insufficiency: The urine flow rate following
furosemide is roughly proportional to the creatinine clearance.
Adequate diuresis is possible even at creatinine clearances as
low as 10-15mL/min. In general, when the collecting system does
not completely fill within one hour after injection (ie:
persistent parenchymal activity) or when the affected kidney has
less than 20% of the total renal function, a prolonged washout
is not be a reliable indicator of obstruction. The Radionuclides
on Nephrology consensus statement recommends that a single
kidney GFR (SKGFR) be used in interpreting the significance of
abnormal T1/2 and renogram curve shapes:
- The negative predictive value for a normal T1/2 and curve shape is high in all cases.
- SKGFR > 15ml/min with an obstructive curve should be interpreted as obstructed.
- SKGFR < 15ml/min with obstructive or indeterminate curve should be interpreted as indeterminate, not partially obstructed.
- SKGFR > 15ml/min with an indeterminate curve should be interpreted as probably obstructed.
2- Volume and compliance of the dilated collecting system
Massive hydronephrosis can result in apparently abnormally prolonged clearance due to a reservoir effect [11]. T1/2 is directly proportional to the collecting system volume:
T1/2 = k (Collecting system volume)/flow rate
3- Distortion of true activity in the collecting system
Background activity or prolonged cortical retention of tracer can
both affect the calculated clearance time (ie: retained
parenchymal activity will wash into the collecting system and
artifactually prolong the T1/2).
4- Functional Obstruction
a. Distended Bladder
A distended bladder may prolong clearance due to the increased intravesicular pressure which will slow collecting system drainage or the presence of vesicoureteral reflux. An indwelling foley catheter will eliminate this effect, as well as permit measurement of urine output and decrease the radiation dose to the gonads. If a catheter is not used, patients should void prior to the initiation of the lasix portion of the exam. A post void image should also be obtained if the bladder is distended at the completion of the lasix portion of the exam [9].
b. Postural stasis
Certain patients may drain completely when erect. It is essential that a standing/upright post-void view be obtained [9]. In children, prone imaging should be performed if there is delayed clearance on supine images. Markedly improved drainage in the prone position makes high grade obstruction unlikely, and is usually associated with preservation of renal function.
REFERENCES:
(1) J Urol 1994;152: 593-5
(2) Semin Nucl Med 1992; Conway JJ. "Well-tempered" diuresis renography: its historical development, physiological and technical pitfalls, and standardized technique protocol. 22: 74-84
(3) J Nucl Med 1996; O'Reilly P, et al. Consensus on diuresis renography for investigating the dilated upper urinary tract. Radionuclides in Nephrourology Group. Consensus Committee on Diuresis Renography. 37(11):1872-6
(4) J Nucl Med 2001; Rossleigh MA. Renal cortical scintigraphy and diuresis renography in infants and children. 42: 91-95
(5) Semin Nucl Med 1994; Valkema R, et al. Sharp peaks in the downslope phase of the diuresis renogram. 24: 350-353
(6) J Nucl Med 2001; Boubaker A, et al. F + 0 renography in neonates and infants younger than 6 months: an accurate method to diagnose severe obstructive uropathy. 42: 1780-1788
(7) J Nucl Med 2003; Moon DH, et al. Value of supranormal function and renogram patterns on 99mTc-mercaptoacetyltriglycine scintography in relation to the extent of hydronephrosis for predicting ureteropelvic junction obstruction in the newborn. 44: 725-731
(8) J Nucl Med 2005; Liu Y, et al. The F+0 protocol for diuretic renography results in fewer interrupted studies due to voiding than the F+15 protocol. 46: 1317-1320
(9) J Nucl Med 2006; Boubaker A, et al. Radionuclide
investigations of the urinary tract in the era of multimodality
imaging. 47: 1819-1836
(10) AJR 2011; Bao J, et al. Key variables for interpreting 99mTc-mercaptoacetyltriglycine
diuretic
scans:
development
and validation of a predictive model. 197: 325-333
(11) Radiographics 2013; Uliel L, et al. Nuclear medicine in the
acute clinical setting: indications, imaging findings, and
potential pitfalls. 33: 375-396
(12) Radiographics 2014; Lamba R, et al. Multidetector CT of
vascular compression syndromes in the abdomen and pelvis. 34:
93-115
(13) J Nucl Med 2014; Taylor AT. Radionuclides in nephrology,
part 2: pitfalls and diagnostic applications. 55: 786-798
(14) Radiographics 2015; Potenta SE, et al. CT urography for
evaluation of the ureter. 35: 709-726
(15) AJR 2018; Wang Y, et al. Safety of administering furosemide
during nuclear medicine renography in patients with sulfonamide
allergies. 210: 866-868
(16) Radiographics 2021; Houat A, et al. Congenital anomalies of the upper urinary tract: a comprehensive review. 41: 462-486