Microsphere brachytherapy- Radioembolization
Liver malignancy:
Transarterial radiation therapy
with 90Y-microspheres is another potential treatment
method for hepatoma patients with low hepatic reserve [1] and in
patients with metastatic disease to the liver [5]. The dual
blood supply to the liver provides a natural selectivity for
tumor therapy [3]. Tumors generally derive their blood supply
from the hepatic artery, while the normal liver parenchyma is
perfused via the portal venous system [3]. When injected via a
branch of the hepatic arterial system, the microspheres
preferentially lodge in the periphery around the tumor [3]. The
preferential deposition of the microspheres in the tumor,
maximizes tumor irradiation while sparing adjacent liver
parenchyma [5]. After implantation, the 90Y-microspheres
remain permanently in place [3].
For patients with
hepatocellular carcinoma, current data suggest that
radioembolization is best placed after the failure of TACE in
the early intermediate-stage HCC or in patients with diffuse
disease (> 4 tumors) or large tumors (>5cm) [26].
Yttrium-90 can be produced via nuclear reactor production or from a 90Sr/90Y generator [4]. Yttrium-90 (half-life 64.1 hours [2.7 days]) decays to stable Zirconium 90 (90Zr) via a 2.28 MeV maximal energy beta-particle and an accompanying antineutrino 99.98% of the time (it is essentially a pure beta emitter) [3,13,18]. The mean energy is 0.94 MeV which corresponds to a maximum range of 11 mm in tissue, a mean path of 2.5 mm, and a X90 (radius of a sphere in which 90% of the energy is deposited) of 5.3 mm (corresponding to 50-200 cell diameters) [3,6,9,18]. When the beta particles interact with liver parenchyma, bremsstrahlung radiation is emitted and can be used for limited indirect imaging [18]. An alternative minor decay route (32 disintegrations per million) is by internal pair production in which 90Y decays to an excited state of 90Zr which then emits a positron (maximum energy 800 keV) [18]. The interaction of the emitted positron produces two 511 keV photons that can be imaged using a PET scanner [18]. 90Y microspheres can deliver an intratumoral dose of 100-150 Gy [6]. As a rule of thumb, 1 Gbq (27 mCi) of uniformly dispersed 90Y delivers an absorbed dose of about 50 Gy (5000 rads) [3,18].
Two types of microspheres are
available for clinical use- resin and glass microspheres [4].
Resin microspheres (SIR-spheres) are non-degradable polymer
beads between 20-60 ?m in diameter and are loaded with
90Y at a specific activity of 40-70 Bq (50 Bq [41]) per
sphere (the radioisotope is bound to the surface of the resin
microsphere [34]) [4,13,18]. Glass microspheres (TheraSpheres)
measure between 20-30 ?m in
diameter and are loaded with 90Y at a
specific activity of 2400-2700 Bq (2500 Bq [41]) per sphere (the
radioisotope is incorporated into the glass matrix [34]) [4].
Resin microspheres have a lower specific activity than glass
microspheres (the specific activity of the resin microspheres is
50 times lower than that of TheraSpheres- approximately 50
Bq/microsphere versus 2,500 Bq/microsphere [18]) [13,34]. But
resin microspheres also have a lower specific gravity and a higher
number of particles per treatment and therefore a greater embolic
effect for any given desired activity (i.e.- more particles can be
delivered which can result in more uniform particle distribution
in the tumor- and this may be important for larger or
hypervascular tumors [42]) [13,34,42]. Glass microspheres remain
fixed in the liver and are not found in any body fluid, whereas
trace amounts of 90Y activity may be excreted in the
urine for the first 24 hours following treatment with resin
microspheres [4]. It has been suggested that because of glass
microspheres low embolic load, they are less likely to limit the
prescribed activity of 90Y, while the heavy embolic
load of resin microspheres can result in arterial stasis, limiting
the actual 90Y dose (i.e.- inability to deliver
the entire prescribed dose) [36]. Stasis can also result in reflux
of particles into off target arteries [42]. It has been reported
that early stasis can be seen in approximately 20% of patients
that are treated with resin microspheres [42]. Early stasis can be
seen even more frequently (up to 38%) in patients who have
received multiple prior lines of chemotherapy, including hepatic
arterial infusion pump chemotherapy [42]. The likelihood for early
stasis can be reduced by delivering the resin microspheres using
5% dextrose or 50% contrast material and 50% saline (note this
method is not recommended by the manufacturer, but does also
permit fluoroscopic monitoring of the flow rate during delivery),
instead of sterile water [42]. Glass microspheres are delivered in
saline which precludes angiographic monitoring during infusion
[42].
166Ho-microspheres have been approved for clinical use in the European Union [48]. 166Ho (Holmium) has a half-life of 26.8 hours and a beta emission (max 1.85 MeV) [50]. The agent also emits an 81 keV gamma ray which can be used for imaging purposes [49]. There is a tumor absorbed dose-patient response relationship, with higher tumor absorbed doses associated with complete response [48]. In one study, the mean tumor absorbed dose was 232 Gy in complete responders, 147 Gy in patients with stable disease, and 116 Gy in patients with progressive disease [48]. Patients with an objective response have been shown to exhibit significantly higher overall survival than non-responders (19 months versus 7.5 months) [48].
Pre-procedure antibiotics are often given prior to
radioembolization (a single dose of cefazolin IV), although
infectious complications are rare [42]. However, for patients with
a biliary anastomosis or incompetent sphincter, broad spectrum
antibiotics are recommended starting before the procedure and
continuing for 5 days following the intervention [42]. To reduce
the risk of gastric and duodenal ulcers, a proton pump inhibitor
can be administered before and for 1-4 weeks after treatment [42].
To reduce post-radioembolization syndrome (nausea, fatigue, and
pain), an antiemetic and a steroid can be given before the
procedure [42].
The average dose to the tumor
is 100-600 Gy and less than 1% of the normal liver receives more
than 30 Gy (if the healthy liver absorbs a dose higher than 30
Gy, the risk of irreversible liver damage limits the overall
effectiveness of the treatment) [3,6,16]. Other reported dose
limits for the liver are 50 Gy to one third or 35 Gy to
two-thirds of the whole liver volume [16]. Other authors report
that for lobar radioembolization the maximum tolerable normal
liver absorbed dose is less than 70 Gy when using resin
microspheres and less than 120 Gy when using glass microspheres
[25,38]. For safe treatment, the dose to the lungs should be
less than 30 Gy (other authors report the dose must not exceed
30 Gy to 20%, or 15 Gy to 30% of the whole lung volume [16])
[15]. Unfortunately, activity planning for radioembolization is
inexact and this can contribute to the non-response rate and to
hepatotoxicity (which can occur in up to 20% of patients) [24].
For bilobar disease, the left and right lobe are typically
treated in separate sessions 4-8 weeks apart [42]. Treatment of
the entire liver in a single session is associated with a higher
rate of liver failure [42].
For glass microspheres, there
is a strong correlation between tumor response and the dose
absorbed by the tumor [15]. If the lesion-absorbed dose is too
low, the procedure will be ineffective [16]. A response with
improved progression free survival can be predicted using a
tumoral threshold dose of 205 Gy or more [15]. Other authors
report that when an average dose of 120 +/- 20 Gy can be
delivered to the liver lobe bearing the tumor lesions, median
survival ranges from 7.1-21 months in patients with HCC, and
from 6.7 to 17 months in patients with colorectal liver
metastases [16].
For resin microspheres, in one
study, the 1-year survival for patients whose tumors received a
dose of more than 55 Gy was 100%, whereas the survival was 24%
if the dose was below 55 Gy [24]. A tumor dose of over 77 Gy was
associated with a 2 year survival of 100%, whereas the survival
was 10% when the dose was below 77 Gy [24]. In another study
using 90Y-microspheres, overall survival was
greatest in patients with tumor absorbed doses of 100 Gy or
higher [51]. Other authprs have suggested a 120 Gy threshold for
target-tumor radiation absorbed dose with resin microspheres [52].
The treatment is well tolerated, does not typically induce significant hepatic or pulmonary toxicity, and can be administered on an outpatient basis because post embolization syndrome is minimal [1]. Also- the treatment results in very little radiation exposure to heath care workers or family members [4].
Eligible patients should be non-surgical candidates with adequate
liver function (Child-Pugh score less than or equal to B7) [5,32].
Relative
contraindications include main portal vein thrombosis (although
patients with PVT can be treated safely and effectively with a
meaningful increase in overall survival, particularly for
patients with tumors smaller than 5 cm and Child-Pugh class A
patients [43]), bile duct abnormalities or stents, a serum
bilirubin > 34 umol/L (2mg/dL), a leukocyte count < 200 or
a platelet count < 60,000, and a GFR < 35 [32].
Absolute contraindications
include extensive and untreated portal hypertension, significant
extrahepatic disease, a life expectancy of less than 3 months,
active hepatitis, and unacceptable shunting on Tc-MAA
pre-treatment imaging (uncorrectable flow to the GI tract observed or pulmonary
shunting with more than 30 Gy estimated dose to be delivered to
the lungs in a single dose, or more than 50 Gy in cumulative
doses) [5,32]. Main portal vein thrombosis is also a
contraindication, but treatment may be considered on a case by
case basis [34]. Age is not a contraindication to treatment and
has not been shown to alter prognosis [32]. Prior surgical liver
resection is not a contraindication, but surgical procedures
involving the biliary tract may be a risk factor for infectious
complications [32]. Other contraindications include
poor liver function (bilirubin > 2 mg/dL; albumin < 3 gm/dL;
uncontrolled ascites) and poor performance status (Eastern
Cooperative Oncology Group performance status > 2) [42].
Caution is advised in patients who have a bilirubin level of 1.5
mg/dL (unless super-selective embolization is performed) and in
patients with limited hepatic reserve [34].
Bevacizumab is typically withheld for at least 2 and ideally 4
weeks before mapping angiogram and radioembolization procedures,
although optimal timing is unknown [42]. Bevacizumab interferes
with wound healing, may result in hepatic artery dissection, and
increases the risk of stasis being reached- resulting in inability
to deliver the entire dose, as well as possible reflux and
gastroduodenal ulceration [42].
Mapping lung shunting: Due to disorganized angiogenesis
within metastatic lesions, particles may potentially pass through
intratumoral hepatic shunts and lodge in the capillaries of the
lungs [40]. Prior to treatment, vascular mapping and a
hepatopulmonary shunt fraction should be determined using Tc-MAA
and planar or SPECT-SPECT/CT imaging [6,18]. Vascular mapping is
very important because anatomic variants of the hepatic arterial
vasculature are common [32]. The normal hepatic arterial supply
originates from the celiac trifurcation from which the common
hepatic artery arises [32]. The common hepatic artery becomes the
proper hepatic artery after the gastroduodenal artery branches off
[32]. The proper hepatic artery branches into the right and left
hepatic arteries [32].
Prior to injection, the Tc-MAA syringe should be gently tilted to
agitate and re-suspend the MAA particles- this will minimize
clumping of the particles [18]. The injection should be given
slowly to avoid streaming [18]. The typical dose is 5 mCi (185
mBq) suspended in normal saline- this can be divided between the
right (3mCi) and left (2 mCi) lobes if whole liver imaging is
performed [18]. Tc-MAA can begin to significantly breakdown into
free technetium and varied-sized particles within 75 minutes of
administration [33]. Scintigraphy should be performed within one
hour of MAA injection to prevent false positive extrahepatic
activity due to free technetium (free tech usually produces
diffuse gastric uptake and can also be seen in the thyroid gland
and urinary system, while pathologic uptake is usually focal)
[11,18,33]. The shunt fraction to the lungs is calculated by
dividing the total lung counts by the sum of the lung and liver
counts [6].
For resin microspheres the amount of administered activity is
adjusted on the basis of the calculated shunt fraction [41]. For a
shunt fraction of < 10%, there is no reduction in dose [41]. A
shunt fraction of > 20% is a contraindication to therapy
with resin microspheres [6]. Treatment guidelines recommend a 20%
decrease in administered radioactivity for patients with a lung
shunt fraction between 10-15%, and a 40% decrease in administered
activity for patients with lung shunt fractions between 15-20%
[40]. However, reducing the administered activity can result in
sub-therapeutic treatment dosing [40]. The degree of liver
cirrhosis has been shown to influence the intra-hepatic 90Y
distribution [47]. Compared to patients with
Child-Pugh A liver disease, those with Child-Pugh B have
demonstrated increased rates of non-target 90Y
delivery and higher lung shunt functions [47]. This may be related
to cirrhosis associated structural changes with portal
hypertension, arterioportal, and hepatovenous shunting [47]. A
reduced dose delivered to the tumor results in lower response
rates [47].
Because glass microspheres contain more activity per microsphere,
a shunt fraction of 10% should be used [6]. Other authors suggest
that for glass microspheres the upper limit of allowed activity
shunted to the lungs is 16.5 mCi, calculated by multiplying the
lung fraction shunt percentage by the planned therapeutic activity
[41].
The highest tolerable accumulated absorbed dose to the lungs is
defined as 30 Gy after a single treatment and up to 50 Gy after
repeated treatments [32]. Bevacizumab is an antiangiogenic agent
that is being incorporated into many metastatic colorectal cancer
treatment regimens and may be expected to decrease the disorderly
angiogenesis of tumor growth and result in a lower lung shunt
fraction [40].
If other sites of extrahepatic activity are identified on Tc-MAA
imaging, coil embolization of the culprit vessel, or a more distal
position of the catheter/superselective catheterization (such as
placing the microcatheter distal to the cystic artery) can be used
during the procedure [32,33]. In the absence of significant
extrahepatic activity, the other dosimetric limitation is total
absorbed radiation dose in the healty liver parenchyma [32]. A
nontumor liver dose of less than 70 Gy (or 50 Gy in cirrhotic
livers) has been proposed [32].
SPECT imaging following MAA administration leads to more accurate
calculation of lung shunting (the lung shunt absorbed dose is
typically overestimated by planar imaging compared to SPECT [32])
and SPECT is more sensitive and able to detect shunting in a
larger number of patients [8]. SPECT/CT has an even higher
sensitivity, specificity, and accuracy for the detection of
abnormal shunting/extrahepatic sites of activity [8,11,18]. In a
study comparing planar, SPECT, and SPECT/CT imaging, the
sensitivity for detecting extrahepatic shunting was 32%, 41%, and
100%, respectively [11]. SPECT/CT permits better localization of
extrahepatic activity- especially for areas such as the
gallbladder wall and duodenum that may lie in close proximity to
the liver [11]. The therapy plan may be changed in up to 29% of
patients based upon the SPECT/CT exam findings [11]. Gastric
activity is also more commonly seen on SPECT imaging, but can
sometimes occur secondary to free pertechnetate [14]. The
administration of sodium perchlorate prior to tracer
administration has been suggested as a means to decrease free
pertechnetate activity in the stomach [14]. Focal increased MAA
activity in the falciform artery, phrenic artery, duodenum,
gastric lumen, or anywhere along the GI tract is concerning for
extrahepatic shunting due to hepaticoenteric arterial
communications [33]. These vessels include the falciform,
accessory or left phrenic, right, or accessory gastric arteries
(from the left hepatic artery), supraduodenal, retroduodenal, and
accessory right hepatic artery feeding segment 6 (from the
gastroduodenal artery) [33].
One might expect a higher response to radioembolization of tumors
that demonstrate high MAA uptake compared to those with only low
MAA activity [30]. Interestingly, in most instances the degree of
intra-tumoral uptake of Tc-MAA does not appear to predict the
likelihood for Y90 uptake or response to Y90 resin microspheres
and treatment should not be withheld from patients with liver
tumors or colorectal liver metastases lacking intratumoral Tc-MAA
accumulation [19,35]. In one study, more than 60% of lesions with
a pre-therapy uptake lower than healthy liver tissue showed uptake
following radioembolization [35]. This discrepancy may be related
to the number of particles used for the MAA study (approximately
150,000), compared to the number of resin particles used for
treatment (23 million - 300 times more) [19]. Therefore, even
lesions that appear hypovascular on Tc-MAA imaging, may receive a
sufficient number of particles to have a therapeutic effect [19].
However, MAA uptake in hepatocellular carcinoma has been shown to
be predictive of response following radioembolization with glass
microspheres with responding tumors having almost double the MAA
uptake of nonresponding HCC [30]. Personalized dosimetry based on
the MAA scan in a select group of patients with HCC and portal
vein thrombosis have been shown to result in prolonged overall
survival following treatment with glass microspheres [31]. In
general, the lesions which generally have the highest MAA activity
include HCC, NET, and cholangiocarcinoma [30]. In patients with
metastatic colorectal cancer, a tumor-to-normal liver uptake ratio
of greater than 1 has been correlated with a good metabolic
response [42].
Some authors suggest that MAA distribution does not accurately
predict the 90Y
distribution (for resin microspheres) [21]. In one study, up to
68% of segments demonstrate a greater than 10% difference
between MAA and 90Y activity [21]. These
discrepancies may be related to slight differences in catheter
positioning, physiologic variance in hepatic blood flow, and
morphologic differences between MAA particles and 90Y microspheres [21].
Combined Tc-MAA and Tc-sulfur
colloid imaging can provide information regarding treatment
distribution and functioning liver tissue that can aid in proper
dose determination (this method assumes that the intact
reticuloendothelial function defined by the Tc-SC scan also
corresponds to regions of intact hepatocellular function) [24].
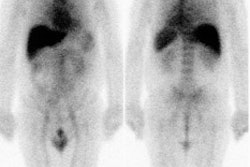
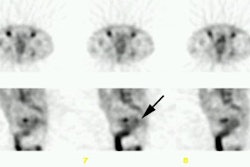
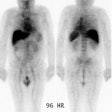
| Shunting to lung: The patient below was
referred for Y90-microshere therapy. A pre treatment TcMAA
hepatic arteriogram revealed a severe intrahepatic
arteriovenous shunt with a shunt fraction of 87%. Note the
intense lung activity. |
 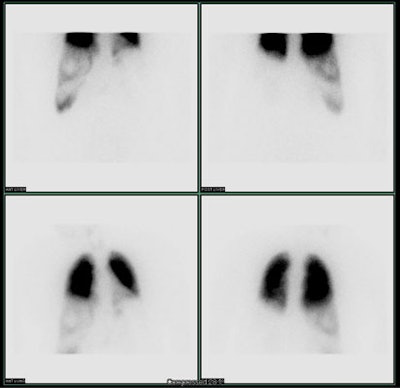  |
Dosimetric assessment and treatment activity determination:
The maximum activity to be injected to the patient is determined
using one of three methods for resin microspheres - the body
surface area, the empiric model, or the partition model- or the
volume-based model for glass microspheres [27,32,44].
The empiric model recommends exclusively three values of activity
based on tumor burden in the liver [44,46]. For tumor involvement
of more than 50% of the liver, 3.0 GBq of activity is recommended;
for 25-50% 2.5 Gbq is recommended; and for less than 25% tumor
involvement 2.0 GBq is recommended [46]. This method has been
replaced by the body surface area method [46].
The body surface area (BSA) is based on patient surface area
(calculated from the patients weight and height) and percentage of
liver tumor involvement (the injected activity is adjusted
depending on tumor burden and the patients physical
characteristics), but neglects the tumor-to-normal liver uptake
ratio in [27,44]. Additionally, this simple method does not
incorporate tumor mass, a tumor-absorbed dose, and it does it
account for inter-individual differences in microsphere
distribution and as a result, the achieved tumor-absorbed dose may
be suboptimal and impair treatment efficacy [37].
The partition model is a dosimetric model based on the MIRD
approach in which limit values on mean absorbed doses to organs at
risk (the lungs and non-tumoral liver) are considered [27]. A
noncompartmental MIRD model can be used for glass microspheres and
a compartmental MIRD model can be used with either glass or resin
microspheres [46]. With the MIRD method, the tumor-to-nontumor
tissue ratio is used to express the relative distribution of
Tc-MAA by determining areas of interest in healthy and tumoral
liver tissue at SPECT image acquisition [46]. The aim is to
deliver a tumoricidal dose to the tumor while preserving safe
limits of radiation to normal liver parenchyma and the lungs [46].
The recommended safe dose limits are 70 Gy for non tumor liver
tissue (< 50 Gy in cirrhotic livers) and 30 Gy to the lungs in
a single injection, or 50 Gy total in subsequent treatments [46].
Although this method is more accurate and personalized and
permits better therapy selectivity, its main drawback is the
underlying assumption of homogeneous activity within regions of
interest (uniform dose distribution in tumor) [27,44].
It has been shown that metastases with a higher tumor-absorbed
dose have a better metabolic response and this is associated with
prolonged overall survival [37].
To decrease the risk of stasis and reflux during administration
the microspheres should be given using a slow and pulsatile
injection technique [33]. Because Y90 is bound to the resin
micospheres through an ion exchange mechanism, sterile water
(which is nonionic) has traditionally been used for administration
[33]. However, sterile water can remove arterial endothelium and
cause vessel constriction/spasm [33]. Using glucose 5% solution (a
physiologic isotonic nonionic solution) may help to prevent
endothelial injury and vasoconstriction and reduce the need for
periprocedural pain medication [33].
Immediately following embolization, planar or SPECT imaging should be performed to detect bremsstrahlung radiation (produced by interaction of the emitted beta-particles with adjacent tissue) and confirm intrahepatic/tumor deposition of the microspheres [6]. Although SPECT imaging has the potential to provide the most accurate depiction of tracer activity, the wide range (0-2.3 MeV) and continuous nature of the 90Y bremsstrahlung photon spectrum prohibit the use of simple energy window-based scatter rejection and correction techniques, and require compensation for collimator and detector related imaging-degrading artifacts such as collimator scatter, lead x-rays, septal penetration, and partial energy deposition in the crystal [22]. Monte Carlo-based modeling can substantially improve image quality and quantitative accuracy of 90Y bremsstrahlung SPECT images [22]. Recent studies also suggest that there is a low incidence of positron decays associated with Y90 that can be detected on PET/CT [12], but this requires use of a time-of-flight PET/CT to obtain images with sufficiently high quantitative accuracy if dosimetric evaluation is going to be performed [22].
Results:
Hepatocellular carcinoma:
Up to 79% of patients with HCCa can show a positive tumor
response [6] and prolonged time to progression compared to
chemoembolization [41]. The greater the amount of radiation
delivered to the tumor, the better the response rate [6]. In one
study, a mean tumor dose of 215 Gy was noted in responders
(partial or complete) vs 167 in non-responders [32].
Metastatic disease:
Approximately 45% of patients with colorectal cancer develop
liver metastases [39]. These metastases are synchronous (present
at diagnosis) in 25% of patients or metachronous in 20% of
patients [39]. The only potential curative treatment is liver
resection, which is associated with 5-year survival rates of
20-58% [39]. However, only approximately 15% of patients are
eligible to undergo resection [39]. In colorectal cancer patients,
modern chemotherapy regimens and biologic agents have
significantly prolonged the median overall survival of patients
with liver metastases to approximately 29-32 months [36]. However,
once hepatic metastases become chemorefractory, survival is poor
and typically between 4 to 5 months [36]. 90Y
radioembolization can be used for treatment in chemorefractory
patients.
For patients with metastatic colorectal cancer, microsphere
embolization has been associated with better median survival
compared to chemotherapy alone (approximately 10.5-10.6 months for
both resin and glass microspheres) [6,36]. In one study of
patients with unresectable liver metastases, microsphere therapy
produced a complete response in 2% of patients and a partial
response in 43% [7]. Some decrease in tumor size was noted in 87%
of patients [7]. Survival following treatment was best for
patients with four or fewer lesions and those with neuroendocrine
tumors [7]. In patients with metastatic colorectal adenocarcinoma,
a lung shunt fraction of more than 10% has been shown to be an
independent predictor of significantly decreased survival
following embolization [40]. Other factors associated with shorter
survival include a ECOC performance status of greater than or
equal to 1, low albumin level, presence of extrahepatic
metastases, lymphovascular invasion of the primary tumor, CEA
level of greater than 62 ng/mL, KRAS mutant tumors, and greater
than 25% tumor involvement of the treated liver volume [42].
For treatment of metastatic
neuroendocrine tumor, a meta-analysis found an objective
response rate (CR or PR) of 50% and a weighted avergae disease
control rate (CR, PR, or stable disease) of 86% [29]. The
response rate was overall slightly lower for patients with
metastatic pancreatic neuroendocrine tumor (pancreatic
neuroendocrine tumors tend to be more aggressive and small bowel
primaries have a nearly 2 fold higher 5 year survival rate
[29]).
Radioembolization in combination with systemic chemotherapy can
be used to increase median survival in patients with metastasis
confined to the liver [17]. A literature review found disease
control rates (complete response, partial response, and stable
disease) ranged from 29-90% for 90Y
radioembolization and from 59-100% for radioembolization combined
with chemotherapy [23]. Survival at 12 months ranged from 37-59%
for Y90 treatment alone, and from 43-74% for Y90 with chemotherapy
[23]. The article also noted that there is a large amount of
heterogeneity in the patient populations being studied with
regards to disease extent, prior therapies, patient performance
status, and criteria used to determine tumor response [23].
Standard RECIST criteria can
underestimate response following treatment [39]. Due to edema or
inflammation, responding tumors may increase in size following
treatment (before 30 days) [46] - although the responding
lesions should show evidence of necrosis [2] and decreased
attenuation [17]. In general- the presence of necrosis (and
decreased attenuation) is a better indicator of response
[5,10,17]. Some authors recommend a wait of 3 months before
assessing tumor response [46]. Ring enhancement about the
lesions may also be seen following therapy and often represents
granulation tissue or fibrosis rather than neoplastic tissue-
the ring enhancement may persist for months [5,6]. This rim of
enhancement is usually smooth and less than 5 mm thick and can
be seen in about one-third of treated lesions (an enhancing
peripheral nodule is more concerning for residual tumor) [46].
Variable areas of necrosis and residual enhancement do not have
predictive value if they are present during the early followup
period (30 days), however, persistence after 90 days most likely
represents residual disease [46]. Hypertrophy of the
non-embolized liver lobe may also occur following treatment [5].
Modified RECIST criteria used
for therapy response evaluation include- complete response-
disappearance of any intra-tumoral enhancement in all target
lesions; partial response- > 30% decrease in the sum of the
diameters of the viable target lesions; stable disease; and
progressive disease - > 20% increase in the sum of the
diameters of viable target lesions [46]. The Choi criteria
define a partial response as a 10% reduction in size or a 15%
reduction in the attenuation of treated lesions during the
portal venous phase of imaging [46].
PET imaging is able to better detect lesion response compared to conventional imaging [2,5]. Responding lesions will demonstrate decreased tracer uptake and SUVmax values [10]. However, some authors suggest that changes in the total metabolic volume and total lesion glycolytic rate are better predictors of survival than changes in SUVmax or RECIST 1.1 criteria [20]. Patients demonstrating a response on FDG PET imaging have been shown to have longer survival periods compared to patients with non-responding lesions [17]. FDG PET response is best assessed 12 weeks following radioembolization [17]. However, PET is limited in its ability to detect small tumors [2].
Common side effects include
fatigue, self-limited abdominal pain, nausea, fever, anorexia,
and diarrhea [7]. The embolic effect of resin microspheres can
sometimes lead to acute ischemic pain during injection, however,
it has been shown that when 5% glucose is used instead of
sterile water for injection, there is less pain, less stasis,
and more efficient administration [32].
Complications:
- Post-radioembolization
syndrome: Symptoms include nausea, fatigue, pain, and low grade
fever that last for 1-2 weeks following treatment [42].
- Radiation cholecystitis: Due to microspheres entering the cystic artery [5]. Radiation cholecystitis can occur in up to 23% of patients and liver edema up to 42% of patients [2]. Most patients are asymptomatic and imaging findings generally improve with conservative treatment, however, cholecystectomy may be required (in one article only 0.8% of patients developed clinically significant radiation-induced cholecystitis [28] and another indicated that fewer than 1% of patients with radiation-induced cholecystitis require surgical intervention [46]) [2,5]. Radiation cholecystitis appears as GB wall thickening, enhancement, and discontinuity on CT imaging [2].
- Radiation hepatitis/
radioembolization induced liver disease (REILD): Radiation
induced liver disease can develop 4-8 weeks after
radioembolization (although more delayed hepatic toxicity can
also occur) in 4-9% of patients, but can be seen in up to 20% of
patients, particularly those that have undergone pretreatment
with chemotherapeutic agents [42,46]. Risk factors for
radioembolization induced liver disease include prior
chemotherapy, lower tumor burden, high baseline bilirubin,
younger age, low body mass index, whole liver radioembolization,
non-HCC pathology, and cirrhotic liver disease [32,42].
Jaundice, elevated LFTs (bilirubin and alkaline phosphatase),
and ascites in the absence of tumor progression or bile duct
dilatation are the main symptoms of radioembolization induced
liver disease [32].
Histology shows venoocclusive
disease in severe cases [42]. Treatment
for radiation hepatitis/radiation induced liver disease is
usually medical (steroids and anti-inflammatory drugs) [5].
Radiologic findings include intraparenchymal
edema and hepatomegaly [5].
- Biliary necrosis and biloma: Potential biliary complications such as cholangitis and biloma can be seen [46]. Unlike the liver which has a dual blood supply, the biliary tree has only a single blood supply- the peribiliary plexus [46]. Acute biliary necrosis is usually seen as small cystic structures adjacent to a portal venous branch within the distribution of an embolized artery or in clusters around a treated tumor [46]. Leaking bile can accumulate to form a biloma [46]. Interestingly, compared to non-cirrhotic livers, cirrhotic livers have a lower risk of biliary necrosis after radioembolization due to hypertrophy of the peribiliary pelxus [46].
- Radiation pancreatitis
- GI tract ulceration- when microspheres enter into the GI circulation via the gastroduodenal artery, right gastric artery, or other vessels supplying the stomach and small bowl, local radiation can result in ulceration [5]. 90Y induced ulcers in the stomach or duodenum can be resistant to medical therapy and surgery may be required [11]. Prophylactic embolization of the gastroduodenal artery, right gastric, and other extrahepatic vessels is recommended by some authors because the risks of reflux outweigh the risk of embolization of these vessels [6,11]. Because these vessels and the organs they supply can revascularize quickly, the embolization should be performed in close proximity to the time of the planned microsphere therapy [11].
- Hepatic biloma or abscess (particularly in patients with incompetant ampulla of Vater) [5].
- Radiation pneumonitis- due to
tumor-associated arteriovenous shunting [6]. A hepatopulmonary
shunt fraction should be determined prior to treatment to
prevent pulmonary toxicity [6].
- Infection- infectious
complications such as liver abscess and cholangitis following
TARE are rare in the setting of an intact ampulla of Vater
(approximately 0-2%) [45]. However, the risk for infection has
been shown to be much greater in patients with biliary enteric
anastomosis or stents and drains spanning across the ampulla of
Vater- between 10-48% [45]. Infection risk has been shown to
remain elevated despite antibiotic prophylaxis [45] and the risk
for infection appears to be increased with the use of glass
microspheres [45].
Repeat Radioembolizations:
In patients with advanced liver
tumors, repeat radioembolization can be performed safely using a
sequential lobar approach with 4 to 6 weeks between treatment
sessions and proper pre-treatment patient selection (exclusion
of patients with bilirubin levels exceeding 30 umol/L) [26].
REFERENCES:
(1) AJR 2007; Keppke AL, et al. Imaging of hepatocellular carcinoma after treatment with Yttrium-90 microspheres. 188: 768-775
(2) AJR 2007; Miller FH, et al. Response of liver metastases after treatment with Yttrium-90 microsphers: role of size, necrosis, and PET. 188: 776-783
(3) AJR 2007; Welsh JS. Radiographically identified necrosis after 90Y microsphere brachytherapy: a new standard for oncologic response assessment? 188: 765-767
(4) J Nucl Med 2007; Gulec SA, Siegel JA. Posttherapy radiation safety considerations in radiomicrosphere treatment with 90Y microspheres. 48: 2080-2086
(5) Radiographics 2008; Atassi B, et al. Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. 28: 81-99
(6) Radiographics 2008; Kalva SP, et al. Recent advances in transarterial therapy of primary and secondary liver malignancies. 28: 101-117
(7) Radiology 2008; Sato KT, et al. Unresectable refractory liver metastases: radioembolization with 90Y microspheres - safety, efficacy, and survival. 247: 507-515
(8) J Nucl Med 2009; Hamami ME, et al. SPECT/CT with 99mTc-MAA in radioembolization with 90Y microspheres in patients with hepatocellular cancer. 50: 688-692
(9) J Nucl Med 2010; Gulec SA, et al. Hepatic structural dosimetry in 90Y-microsphere treatment: a monte carlo modeling approach based on lobular microanatomy. 51: 301-310
(10) Radiology 2010; Tochetto SM, et al. Does multidetector CT attenuation change in colon cancer liver metastases treated with 90Y help predict metabolic activity at FDG PET? 255: 164-172
(11) J Nucl Med 2010; Ahmadzadehfar H, et al. The significance of 99mTc-MAA SPECT/CT liver perfusion imaging in treatment planning for 90Y-microsphere selective internal radiation treatment. 51: 1206-1212
(12) J Nucl Med 2011; Gates VL, et al. Internal pair production
of 90Y permits hepatic localization of microspheres
using routine PET: proof of concept. 52: 72-76
(13) Radiology 2011; Lewandowski RJ, et al. Transcatheter
intraarterial therapies: rationale and overview. 259: 641-657
(14) J Nucl Med 2011; Sabet A, et al. Significance of oral
administration of sodium percholate in planning liver-directed
radioembolization. 52: 1063-1067
(15) J Nucl Med 2012; Garin E, et al. Dosimetry based on 99mTc-macroaggregated
albumin
SPECT/CT
accurately
predicts
tumor
response
and survival in hepatocellular carcinoma patients treated
with 90Y-loaded glass microspheres: preliminary
results. 53: 255-263
(16) J Nucl Med 2012; Traino AC, et al. Radiodosimetric estimates
for radioembolic therapy of liver tumors: challenges and
opportunities. 53: 509-511
(17) AJR 2012; Tochetto SM, et al. Colorectal liver metastasis
after 90Y radioembolization therapy: pilot study of
change in MDCT attenuation as a surrogate marker for future FDG
PET response. 198: 1093-1099
(18) J Nucl Med 2012; Uliel L, et al. From the angio suite to the
gamma camera: vascular mapping and 99mTc-MAA hepatic
perfusion imaging before liver radioembolization- a comprehensive
pictorial review. 53: 1736-1747
(19) J Nucl Med 2013; Ulrich G, et al. Predictive value of
intratumoral 99mTc-macroaggregated albumin uptake in
patients with colorectal liver metatsases scheduled for
radioembolization with 90Y-microspheres. 54: 516-522
(20) J Nucl Med 2013; Fendler WP, et al. Validation of several
SUV-based parameters derived from 18F-FDG PET for
prediction of survival after SIRT of hepatic metastases from
colorectal cancer. 54: 1202-1208
(21) J Nucl Med 2013; Wondergem M, et al. 99mTc-macroaggregated
albumin poorly predicts the intrahepatic distribution of 90Y
resin microspheres in hepatic radioembolization. 54: 1294-1301
(22) J Nucl Med 2013; Elschot M, et al. Quantitative Monte
Carlo-based 90Y SPECT reconstruction. 54: 1557-1563
(23) J Nucl Med 2013; Rosenbaum C, et al. Radioembolization for
treatment of salvage patients with colorectal cancer liver
metastases: a systemic review. 54: 1890-1895
(24) J Nucl med 2013; Lam MGEH, et al. Prognostic utility of 90Y
radioembolization dosimetry based on fusion of 99mTc-macroaggregated
albumin-99mTc-sulfur colloid SPECT. 54: 2055-2061
(25) J Nucl Med 2014; Walrand S, et al. The low hepatic toxicity
per gray of 90Y glass microspheres is linked to their
transport in the arterial tree favoring a nonuniform trapping as
observed in posttherapy PET imaging. 55: 135-140
(26) J Nucl Med 2014; Zarva A, et al. Safety of repeated
radioembolizations in patients with advanced primary and secondary
liver tumors and progressive disease after first selective
internal radiotherapy. 55: 360-366
(27) J Nucl Med 2014; Petitquillaume A, et al. Three-dimensional
personalized Monte Carlo dosimetry in 90Y resin
microspheres therapy of hepatic metastases: nontumoral liver and
lungs radiation protection considerations and treatment planning
optimization. 55: 405-413
(28) J Nucl Med 2014; Sag AA, et al. Yttrium-90 radioembolization
of malignant tumors of the liver: gallbladder effects. 202:
1130-1135
(29) J Nucl Med 2014; Devcic Z, et al. The efficacy of hepatic 90Y
resin radioembolization for metastatic neuroendocrine tumors: a
meta-analysis. 55: 1404-1410
(30) J Nucl Med 2015; Ilhan H, et al. Systemic evaluation of
tumoral 99mTc-MAA uptake using SPECT and SPECT/CT in
502 patients before 90Y radioembolization. 56: 333-338
(31) J Nucl Med 2015; Garin E, et al. Personalized dosimetry with
intensification using 90Y-loaded glass microsphere
radioembolization induces prolonged overall survival in
hepatocellular carcinoma patients with portal vein thrombosis. 56:
339-346
(32) J Nucl Med 2015; Braat AJ, et al. 90Y hepatic
radioembolization: an update on current practice and recent
developments. 56: 1079-1087
(33) J Nucl Med 2015; Gates VL, et al. Intraarterial hepatic
SpECT/CT imaging using 99mTc-macroaggregated albumin
in preparation for radioembolization. 56: 1157-1162
(34) Radiographics 2015; Camacho JC, et al. 90Y
radioembolization: multimodality imaging pattern approach with
angiographic correlation for optimal target therapy delivery. 35:
1602-1620
(35) J Nucl Med 2015; Ilhan H, et al. Predictive value of 99mTc-MAA
SPECT for 90Y-labeled resin microsphere distribution
in radioembolization of primary and secondary hepatic tumors. 56:
1654-1660
(36) J Nucl Med 2016; Hickey R, et al. 90Y
radioembolization of colorectal hepatic metastases using glass
microspheres: safety and survival outcomes from a 531-patient
multicenter study. 57: 665-671
(37) J Nucl Med 2016; van den Hoven AF, et al. Insights into the
dose-response relationship of radioembolization with 90Y-microspheres:
a prospective cohort study in patients with colorectal cancer
liver metastases. 57: 1014-1019
(38) J Nucl Med 2016; Pasciak AS, et al. A microdosimetric
analysis of absorbed dose to tumor as a function of number of
microspheres per unit volume in 90Y radioembolization.
57: 1020-1026
(39) AJR 2016; Shady W, et al. Surrogate imaging biomakers of
response of colorectal liver metastases after salvage
radioembolization using 90Y-loaded resin microspheres.
207: 661-670
(40) Radiology 2017; Narsinh KH, et al. Hepatopulmonary shunting:
a prognostic indicator of survival in patients with metastatic
colorectal adenocarinoma treated with 90Y
radioembolization. 282: 281-288
(41) AJR 2017; Jadvar H. Targeted radionuclide therapy: an
evolution toward precision cancer treatment. 209: 277-288
(42) J Nucl Med 2017; Boas FE, et al. Radioembolization of
colorectal liver metastases: indications, technique, and outcomes.
58: 104S-111S
(43) J Nucl Med 2018; Abouchaleh N, et al. 90Y
radioembolization for locally advanced hepatocellular carcinoma
with portal vein thrombosis: long-term outcomes in a 185-patient
cohort. 59: 1042-1048
(44) J Nucl Med 2018; Kafrouni M, et al. Retrospective
voxel-based dosimetry for assessing the ability of the
body-surface-area model to predict delivered dose and
radioembolization outcome. 59: 1289-1295
(45) Radiology 2018; Devulapalli KK, et al. 90Y
radioembolization for hepatic malignancy in patients with previous
biliary intervention: multicenter analysis of hepatobiliary
infections. 288: 774-781
(46) Radiographics 2019; Spina JC, et al. Expected and unexpected
imaging findings after 90Y transarterial radioembolization for
liver tumors. 39: 578-595
(47) J Nucl Med 2019; Schobert I, et al. Quanitative imaging
biomarkers for 90Y distribution on bremsstrahlung
SPECT after resin-based radioembolization. 60: 1066-1072
(48) J Nucl Med 2020; Bastiaannet R, et al. First evidence for a
dose-response relationship in patients treated with 166Ho
radioembolization: a prospective study. 61: 608-612
(49) J Nucl Med 2018;Prince JF, et al. efficacy of
radioembolization with 166Ho-microspheres in salvage patients with
liver metastases: a phase 2 study. 59: 582-588
(50) J Exp Clin Cancer Res 2010; Smits MLJ, et al. Holmium-166
radioembolization for the treatment of patients with liver
metastases: design of the phase I HEPAR trial. 29: 70.
(51) Radiology 2020; Hermann AL, et al. Relationship of tumor
radiation- absorbed dose to survival and response in
hepatocellular carcinoma treated with transarterial
radioembolization with 90Y in the SARAH study. 296:
673-684
(52) Int J Radiat Oncol Biol Phys 2012; Lau WY, et al. Patient
selection and activity planning guide for selective internal
radiotherapy with yttrium-90 resin microspheres. 82: 401-407