223Ra-dichloride (Xofigo)
- Clinical:
223Ra-dichloride (223RaCl2) is
an alpha (α) emitting bone seeking calcium mimetic that forms
complexes with hydroxyapatite and will accumulate in areas of
increased bone turnover/active mineralization without significant
redistribution of the daughter radionuclides [1,2]. The agent is
prepared from a long-lived 227Ac/227Th
generator and has a physical half life of 11.4 days [1]. 223Ra
has a complex decay scheme into stable lead (93.5% α emission,
< 3.6% beta-particles, and <1.1% as gamma radiation) [4,5].
There are four α particles generated during each decay, resulting
in high energy deposition (28.2 MeV), with 95% of the energy from
the α emissions [1].
Alpha particles are monoenergenic positively charged helium
nuclei (initial kinetic energy between 5-9 MeV) that are about
8000 times larger than beta particles [7,9]. Alpha emitters are
100 to 500 fold more potent than low-LET beta-particle emitters
[2] and can produce 2000-7000 ion pairs per micrometer in water
(one ionization every 2 nm) [11]. Because the diameter of the DNA
double-helix is about 2 nm, the traversal of a single
alpha-particle is enough to induce double-stranded, often
blunt-ended, DNA breaks [11]. Beta-radiation results in fewer than
20 ion pairs per micrometer and the traversal of a single
beta-particle causes only single stranded DNA breaks, whereas
higher absorbed doses are needed to achieve double-stranded
breaks, and then often with cohesive ends [11]. Because alpha
particles have a short range (approximately 0.05-0.1 mm in human
tissue vs several millimeters (0.05-12mm) for beta particles
[7,10]), α-particles deliver a large amount of radiation to target
tissue while relatively sparing normal surrounding tissue [5].
With beta particles, neighboring cells around the targeted cell
are also irradiated (cross-fire effect) which is considered
advantageous for targeting large tumors with a heterogeneous
target distribution, but can have a negative effect on adjacent
normal cells (such as bone marrow cells) [10]. As a result of the
short range of α emissions, there should be less hemotoxicity from
α emitters compared to beta emitters [1]. The high linear energy
transfer of the α particle, compared to a beta emission (80-100
keV/?m (50-230 keV/?m [10]) versus 0.2 keV/?m [7,9]), also results in
greater biological effectiveness with more irreversible
double-stranded DNA breaks per unit absorbed dose that cells
cannot repair and hence the damaged cells succumb to apoptosis
[1,2,7]. The range of the particles (<100 um or a few cell
diameters) is also shorter than the 0.7 cm path of 89Sr
and the 0.33 cm path of 153Sm, which results in less
irradiation to health tissue [1,2,9].
With regards to radiation exposure, a patient of average weight
receiving 3.5 mBq of 223Ra-dichloride will emit
radiation at a rate of 0.35 mSv/hr at one meter immediately
following administration which entails minimal radiation safety
precautions [7]. There are no contact precautions with the agent,
but bathroom precautions are recommended (fecal excretion is the
primary mode of clearance [7]) for one week and include multiple
flushing and area clean up [5].
The agent is rapidly cleared from the blood with less than 1%
remaining at 24 hours [1,4]. Most if the administered activity is
rapidly taken up by bone (61% at 4 hours) [4]. The agent
accumulates in the bone matrix in proportion to the extent of
osteosclerotic bone remodeling [8]. Elimination is mainly through
the GI tract (fecal elimination) with the median being 52%
activity in the bowel at 24 hours [1,4,5]. Despite this, there is
low GI tract toxicity [4]. Urinary excretion is minimal (typically
< 5%) [1]. Limited imaging can be obtained from gamma emissions
from 223Ra (1.1% abundance) and its daughter products
[1,4].
Administration is based on body weight with a dose of 50 kBq/kg
(1.35 uCi/kg) given as repeated injections for 6 doses [1]. For
the first dose, the ANC should be 1.5 x 109/L or
greater, the platelet count 100 x 109/L or greater, and
the hemaglobin at least 10 g/dL or higher [1]. For subsequent
doses, the ANC should be 1.0 or higher and the platelet count
50,000 or higher [1]. The agent should be discontinued if
hematologic values do not recover within 6-8 weeks after the last
administration despite supportive care [1].
Results:
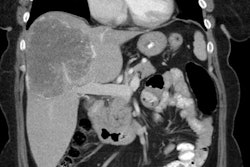
The agent is presently approved for use in patients with bone
metastases from castration resistant prostate cancer (and no known
visceral metastatic disease) [1]. The existence of any visceral
metastases is a contraindication and bone marrow involvement
should also be a contraindication [6]. 223Ra therapy
is effective in providing pain relief which can be seen in 52% of
patients at 1 week, 60% at 4 weeks, and 56% at 8 weeks [1]. Bone
alkaline phosphatase (ALP) is a marker for tumor response in these
patients and a significant decrease in ALP is seen following 223Ra
therapy (a decrease in ALP is more common than a decline in PSA
[5]) [1]. A significant decrease in PSA levels also occurs
following therapy (compare to placebo) [1]. However, lack of a PSA
decrease does not indicate therapeutic failure and therapy should
not be stopped prematurely [5,6]. The decision to discontinue
treatment should be based on clinical symptomatology, therapeutic
tolerability, and radiographic evidence of progression [6]. An
approximately 3.6 month survival benefit has also been
demonstrated in one study (14 vs 11.2 months) [1,5].
Although 223Ra therapy has been shown to improve
survival, there is heterogeneity of response, with some patients
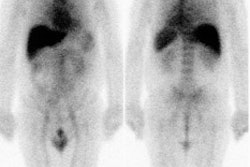
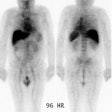
showing a limited or poor response [3]. Some lesions on bone scan
can improve following treatment [8].
The initial skeletal tumor burden identified on bone scan or 18F-flouride
PET/CT has been correlated with worse overall survival following 223Ra
therapy [3,8,12]. Additionally, high tumor involvement of bone
identified on bone scan has also been shown to be associated with
a greater likelihood of inability to complete 223Ra
treatment due to disease progression or cytopenia [12].
Other factors associated with worse survival include an ALP greater than 146 U/L, a pain score greater than 3, skeletal related events, a PSA greater than 10 ng/mL, a hemoglobin less than 12.8 g/dL, visceral or nodal metastases, and older age [3].
Complications/Side effects:
The most common adverse reactions (>10%) are nausea, diarrhea,
vomiting, and peripheral edema [5]. Hematopoietic toxicity is
dose-related with a nadir occurring 2-4 weeks after treatment [1].
Generally, recovery occurs by 24 weeks [1]. It has been suggested
that a greater extent of skeletal metastatic disease on bone scan
is a significant risk factor for developing hematologic toxicity
[8].
Combination use of 223Ra with abiraterone and prednisone (or prednisolone) has been associated with an increased risk for fractures [12].
Long-term followup for the agent is not yet available, but to
date no myelodysplastic syndrome, aplastic anemias, or leukemias
have been observed [1]. In one study, only 25% of patients were
able to complete the entire 6-dose treatment regimen [5].
Advancing soft tissue disease is one of the primary reasons for
cessation of therapy [5].
REFERENCES:
(1) J Nucl Med 2014; Pandit-Taskar N, et al. Bone-seeking
radiopharmaceuticals for treatment of osseous metastases, part 1:
α therapy with 223Ra-dichloride. 55: 268-274
(2) AJR 2014; Wadas T, et al. Molecular targeted α-particle
therapy for oncologic applications. 203: 253-260
(3) J Nucl Med 2015; Etchebehere EC, et al. Prognostic factors in
patients treated with 223Ra: the role of skeletal
tumor burden on baseline 18F-flouride PET/CT in
predicting overall survival. 56: 1177-1184
(4) J Nucl Med 2015; Chittenden S, et al. A phase 1, open-label
study of the biodistribution, pharmaokinetics, and dosimetry of 223Ra-dichloride
in patients with hormone-refractory prostate cancer and skeletal
metastases. 56: 1304-1309
(5) J Nucl Med 2016; Iagaru AH, et al. Bone-targeted imaging and
radionuclide therapy in prostate cancer. 57: 19S-24S
(6) J Nucl Med 2017; Ahmadzadehfar H, et al. 68Ga-PSMA-11
PET as a gatekeeper for the treatment of metastatic prostate
cancer with 223Ra: proof of concept. 58: 438-444
(7) AJR 2017; Jadvar H. Targeted radionuclide therapy: an
evolution toward precision cancer treatment. 209: 277-288
(8) J Nucl Med 2018; Fosbol MO, et al. 223Ra therapy
of advanced metastatic castration-resistant prostate cancer:
quantitative assessment of skeletal tumor burden for
prognostication of clinical outcome and hematologic toxicity. 59:
596-602
(9) J Nucl Med 2018; Poty S, et al. α-emitters for radiotherapy:
from basic radiochemistry to clinical studies- part 1. 59: 878-884
(10) J Nucl Med 2019; Nicolas GP, et al. New developments in
peptide receptor radionuclide therapy. 60: 167-171
(11) J Nucl Med 2020; Kratochwil C, et al. Patients resistant
against PMSA-targeting alpha-radiation therapy often harbors
mutations in DNA damage-repair-associated genes. 61: 683-688
(12) J Nucl Med 2021; Dittmann H, et al. The prognostic value of quantitative bone SPECT/CT before 223Ra treatment in metastatic castration-resistant prostate cancer. 62: 48-54
PMSA radioligand therapy:
PMSA is a type II transmembrane glycoprotein that is
over-expressed in metastatic prostate cancer and the degree of
PMSA expression positively correlates with tumor stage [1,2].
However, the ligand is not specific to the prostate gland and is
expressed on other normal tissues including the normal prostate
epithelium, salivary glands, duodenal mucosa, proximal renal
tubular cells, and neuroendocrine cells in the colon crypts [1,2].
It is also expressed on certain neoplasms including transitional
cell carcinoma, renal cell carcinoma, and colon cancer [1].
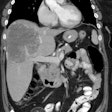
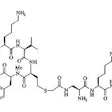
Radionuclide therapy with agents such as 177Lu-labeled PMSA, although not curative, may be considered in patients who have confirmed progressive castrate resistant prostate cancer after exhaustion of approved therapies, including patients previously treated with taxane agents [2,58,95,105]. 177Lu-PMSA is a low molecular weight ligand that binds to the cell surface of prostate carcinoma cells and is transported into the cell by receptor-mediated endocytosis [114]. The 497-keV beta-energy of 177Lu corresponds to a mean and maximum tissue range of only 0.5 and 2 mm (10-50 cell diameters), respectively [58]. The agent has a long effective half-life in both skeletal and soft tissue metastases, approaching the physical half-life of 177Lu and this results in a high mean absorbed tumor dose (the maximum doses obtained in lymph node mets was 468 Gy and in bone mets 260 Gy) [127]. Other authors quote dose estimates between 1.2-47.5 Gy/GBq (mean 13.1 Gy/GBq) [76]. The red marrow dose is low (0.03 Gy/GBq) [76]. Organs at risk are the kidneys (0.5-0.7 Gy/GBQ) and the salivary or lacrimal glands (1.2-28 Gy/GBq) [76].
Treatment: Patients should have confirmed PMSA expression by the tumor and metastatic foci- ideally demonstrated by baseline PMSA-directed imaging (such as with 68Ga-PMSA PET/CT) [2]. Radiation dosimetry has shown that the SUVmean on 68Ga-PMSA imaging correlates with absorbed tumor dose [8].
Patients should have a sufficient bone marrow reserve with a
white blood cell count more than 3000 and a platelet count of more
than 75,000, good renal and liver function (urinary tract
obstruction increases the risk for excessive radiation dose to the
kidneys), and no potentially myelosuppressive therapy for more
than 6 weeks [2]. Patients should have laboratory testing within 2
weeks of planned treatment [2]. A drawback of the agent is that
50% of the administered activity is excreted within 4 hours of
administration and nearly 70% by 12 hours [7].
Most sites use a standard activity of 6.0 to7.4 GBq and an 8 week
interval between treatments [2]. Organs at risk for a critical
radiation dose are the lacrimal, parotid, and salivary glands
(salivary gland dose is 1.4 Gy/GBq) and kidneys (0.75 Gy/GBq)
[1,2]. Ice packs for the salivary glands may be considered to
reduce blood flow [2]. PMSA inhibitors such as 2-(phosphonomethyl)
pentanedioic acid have been proposed to diminish renal PMSA
binding [1].
On ligand binding, PMSA and its bound molecule are internalized
via clathrin-coated pits and subsequent endocytosis [3]. While the
beta-emitting component of 177Lu is utilized for
therapy, the gamma component can be used for imaging [3].
Results:
Between 59-80% of patients will demonstrate some decline in PSA
levels following therapy [85,95,108,109,127].
A decrease in PSA of 50% or more (considered a biochemical
response) can be seen in 32-64% of patients and this decline can
be seen as early as after the first cycle of treatment
[1,2,8,85,95,108,109,127]. Early changes in PSA following therapy
have also been shown to be indicators of long term clinical
outcome [128] with a decline in PSA after the first cycle of
treatment associated with prolonged survival [2]. In another study
following 177Lu-PMSA therapy, a greater than or equal
to 30% decline in PSA values at 6 weeks after treatment was
associated with a longer survival (16.7 months) compared to
patients with stable PSA (11.8 months) or PSA progression (6.5
months) [128]. PSA flare appears to be very uncommon after 177Lu-PMSA
treatment, as opposed to therapy with taxanes which can be
associated with a PSA flare in 20% or more of patients (a PSA
flare is defined as a PSA increase of 25% in the first 6 weeks
after therapy followed by a decline below baseline levels at 12
weeks) [128].
In one study following treatment, the median progression-free survival was 13.7 months and the median overall survival was not reached during a 28 month followup period [127]. In another study, patients that achieved a PSA decline of at least 50% had a median survival of 18.4 months (compared to 8.7 months if the PSA decline was less than 50%) [8]. A meta-analysis has suggested an improved median survival in 177Lu-PMSA treated patients of 2.5 months [109].
There is a significant correlation between whole-body tumor
dose (estimated by pre-treatment 68GA-PMSA imaging)
and PSA response [5]. Patients that receive less than a 10 Gy
tumor dose are unlikely to achieve a fall in PSA of at least 50%
[5]. Due to a tumor sink effect, patients with larger tumor
burdens appear to have lower salivary and renal absorbed doses,
which may permit higher administered activities for therapy [5].
Following treatment, in one study, 56% of patients with
measurable soft-tissue disease achieved an objective response by
RECIST 1.1 [8].
Pain relief can be seen in 33-70% of patients, an improved quality of life in 60%, and improved performance status in 74% [2].
However, one third (20-40%) of patients do not respond despite
the presence of PMSA over-expression on pre-therapy PET scans
[3,76] and therefore, SUVmax on 68Ga-PMSA
imaging is not a predictor of response to therapy [95].
The presence of any visceral metastases or a serum alkaline
phosphatase 220 U/L or higher (an indication of more advanced bone
marrow involvement) are predictive of a poor outcome [2].
177Lu-PMSA retreatment in initial responders who
ultimately relapse has been performed [6,8]. In one study, a PSA
decline of at least 50% was seen in 73% of retreated patients, but
the duration of response was shorter (no increased adverse effects
were observed) [8]. Another study suggested that re-treatment has
a lower efficacy and higher toxicity [6]. In that study, only 38%
of patients demonstrated a 50% PSA decline and the median
progression free survival was 3.3 months, compared to 12.4 months
following the initial course of therapy [6].
Progressive disease following treatment most commonly involves
the marrow (56% of cases) or liver (19% of cases) [8]. Progressive
liver disease in these patients generally demonstrates low PMSA
expression and high metabolic activity [8].
Side effects/Complications:
Serious hematologic adverse events can be seen in up to 12% of patients [2]. Other authors indicate grade 3 to 4 anemia in 10%, thrombocytopenia in 4%, and leukopenia in 3% [1]. A low blood cell count at baseline and diffuse bone marrow involvement pose a risk for serious hematotoxicity [2].
one of the major disadvantages of PSMA-radioligand therapy is the
high accumulation of the agents in non-target tissues [129]. The
PMSA receptor is also expressed in high levels on certain normal
tissues including the proximal tubules of the kidneys, the brush
border of the jejunum, and the salivary and lacrimal glands [114].
It should be noted that chemotherapy with docetaxel can result in toxic side effects in up to 67% of patients, including grade 3-4 myelosuppression in 2-32% of patients [109]. Other toxicities include GI toxicity (0-60%), cardiac toxicity (24-48%), and treatment related death (0.3%) [109].Xerostomia (Dry mouth)- Grade 1 dry mouth has been reported in up to 87% of patients [108] and grade 1-2 in 66% of patients [8], typically self-limiting. External cooling does not appear to reduce tracer uptake by the parotid glands [114].
Orally administered monosodium glutamate (MSG) has been shown to significantly decrease salivary gland, kidney, and other normal-organ uptake, but it also produces a decrease in tumor uptake as well [129].
Nausea- Grade 1-2 transient nausea can be seen in 50% of patients [8,108]. Typically within the first 24 hours of therapy, transient, and manageable with antiemetics [8].
Anemia/Leukopenia/Thrombocytopenia- Grade 3 or 4 anemia (3-10%), neutropenia (1-10%), lymphopenia (32%), and/or thrombocytopenia (2-13%) of patients, respectively [8,105,108].
Renal toxicity- The primary route of 177Lu-PSMA excretion is renal. Grade 1-2 renal injury can be seen in 10% of patients [8]. Grade 3 renal toxicity is a very rare complication [105].
Mannitol infusion is a strategy to reduce renal uptake by acting as an osmotic diuretic, decreasing renal reabsorption [129].
REFERENCES:
(1) AJR 2017; Jadvar H. Targeted radionuclide therapy: an
evolution toward precision cancer treatment. 209: 277-288
(2) J Nucl Med 2017; Fendler WP, et al. 177Lu-PMSA
radioligand therapy for prostate cancer. 58: 1196-1200
(3) J Nucl Med 2017; Hadaschik BA, Boegemann M. Why targeting of
PMSA is a valuable addition to the management of
castration-resistant prostate cancer: the urologist's point of
view. 58: 1207-1209
(4) J Nucl Med 2018; Rathke H, et al. Repeated 177Lu-labeled
PMSA-617 radioligand therapy using treatment activities of up to
9.3 GBq. 59: 459-465
(5) J Nucl Med 2019; Violet J, et al. Dosimetry of 177Lu-PMSA-617
in metastatic castration-resistant prostate cancer: correlations
between pretherapeutic imaging and whole-body tumor dosimetry with
treatment options. 60: 517-523
(6) J Nucl Med 2019; Gafita A, et al. Early experience of
rechallenge 177Lu-PMSA radioligand therapy after
intial good response in patients with advanced prostate cancer.
60: 644-648
(7) J Nucl Med 2019; Kelly JM, et al. Albumin-binding PSMA
ligands: implications for expanding the therapeutic window. 60:
656-663
(58) J Nucl Med 2016; Kratochwil C, et al. PMSA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PMSA-617. 57: 1170-1176
(76) J Nucl Med 2017; Eiber M, et al. Prostate-specific membrane antigen ligands for imaging and therapy. 58: 67S-76S
(85) J Nucl Med 2018; Donin NM, Reiter RE. Why targeting PMSA is a game changer in the management of prostate cancer. 59: 177-182
(95) J Nucl Med 2018; Ahmadzadehfar H, Essler M. Predictive factors of response and overall survival in patients with castration-resistant metastatic prostate cancer undergoing 177Lu-PMSA therapy. 59: 1033-1034
(105) J Nucl Med 2019; Barber TW, et al. Clinical outcomes of 177Lu-PMSA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. 60: 955-962
(108) AJR 2019; Subramaniam RM. Prostate cancer theranosis in clinical practice and in clinical trials- 68Ga-prostate-specific member antigen (PSMA)-11 PET/CT and 177Lu-PMSA-617 therapy. 213: 241-42
(109) AJR 2019; Yadav MP, et al. Radioligand therapy with 177Lu-PMSA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. 213: 275-285
(114) J Nucl Med 2019; Yilmaz B, et al. Effect of external
cooling on 177Lu-PMSA uptake by the parotid glands.
60: 1388-1393
(127)
J Nucl Med 2016; Baum RP, et al. 177Lu-labeled
prostate-specific membrane antigen radioligand therapy of
metastatic castration-resistant prostate cancer: safety and
efficacy. 1006-1013
(128) J Nucl Med 2020; Gafita A, et al. Early prostate-specific
antigen changes and clinical outcome after 177Lu-PSMA radionuclide
treatment in patients with metastatic castration-resistant
prostate cancer. 61: 1476-1483
(129) J Nucl Med 2021; Harsini S, et al. The effects of
monosodium glutamate on PSMA radiotracer uptake in men with
recurrent prostate cancer: a prospective, randomized, doble-blind,
placebo-controlled intraindividual imaging study. 62: 81-87
225Ac-PMSA therapy:
Another agent, the alpha emitter 225Ac-PMSA has also
been studied for therapy. 225Ac is an alpha emitter
with a half life of 9.9 days [1]. 225Ac has six
daughter products (221Fr, 217At, 213Bi, 213Po, 209Pb, and 209Tl)
with several alpha- and beta decays [1]. The agent has been shown
to result in a PSA decline of more than 50% in 63% of patients,
with a median duration of tumor control of 9 months [1]. A PSA
decline of 50% or greater has been shown to be significantly
associated with overall survival (PFS of 15.2 months and OS of
18-20 months) [1].
REFERENCES:
(1) J Nucl Med 2020; Sathekge M, et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving 225Ac-PMSA-617 radioligand therapy. 61: 62-69
(2) J Nucl Med 2018; Kratochwil C, et al. Targeted alpha-therapy of metastatic castrate resistant prostate cancer with 225Ac-PMSA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. 59: 795-802