Empyema:
Clinical:
Empyema represents an infection within the pleural space. Empyema will usually not clear with antibiotic treatment alone and requires drainage via a thoracostomy tube, or thoracotomy. Etiologies include acute bacterial pneumonia (most common), lung abscess, surgery, trauma, and contiguous spread from an adjacent osteomyelitis. Patients with pneumonia can often develop an associated uncomplicated, parapneumonic effusion which can progress to empyema if bacteria enter the pleural space. As the empyema progesses, a fibrin peel coats the surfaces of the visceral and parietal pleura with ingrowth of capillaries and fibroblasts and subsequent thickening- this forms the basis of the split pleura sign [8].For patients with pneumonia, approximately 50% of patients with a gram-negative infection, and 35% of patients with anaerobic infection will develop a parapneumnic effusions, and about 90% of these effusions will progress to empyema. For patients with S. aureus pneumonia, parapneumonic effusion occurs in 40%, and 20% overall will progress to empyema. Although about 50% of patients with pneumococcal pneumonia will have a parapneumonic effusion, only 5% overall progress to empyema. If the empyema extends into the chest wall it is referred to as empyema necessitatis- this is most commonly due to tuberculosis (70% of cases), but actinomycosis, nocardia, and other bacteria can also be responsible [6].
The diagnosis of an empyema by Light's criteria requires one of the following features: gross pus, organisms on gram stain, a pH below 7.0, or a glucose level below 40 mg/dl. In patients with Proteus mirabilis infection, the pH can remain elevated despite infection because of the urea-splitting properties of the organism. Treatment of an empyema consists of percutaneous or surgical drainage. Percutaneous catheter placement by CT or ultrasound guidance is preferred so that the collection is not missed. Catheter drainage of parapneumonic effusions is mandatory for patients that do not respond to antibiotic therapy, or if a low pH (less than 7.0), low glucose (less than 40 mg/dl), high LDH (greater than 1000 IU/L), frank pus, positive gram stain, or positive culture is found at thoracentesis. [1,6]
In patients with long standing empyemas, organization of the infection can result in extensive pleural fibrosis or "pleural peel." This can result in lung restriction and decreased lung volume (trapped lung).
Therapy:
Percutaneous drainage will usually necessitate the use of a large bore catheter such as a 10 to 16 French tube. Once a percutaneous drain is in place, it should be flushed with 10 to 50 ml of saline every 8 hours to maintain catheter patency. If drainage output is minimal (less than 10 ml daily), the patient has clinically improved (decreased WBC count and fever), and the CT scan reveals no further collection, the catheter can be removed.Intracavitary urokinase can be used to promote lysis of septae within loculated effusions. Urokinase is a potent activator that cleaves plaminogen to the active protease plasmin, which in turn degrades fibrin- thereby producing dissolution of fibrinous complexes- it is fibrin deposition which causes locule formation within an empyema. Typically 80,000 to 150,000 IU of urokinase is diluted in 50-100 ml of saline and injected through the percutaneous catheter which is then clamped for 30 to 60 minutes (up to 12 hours). Retreatment intervals vary between 8 to 24 hours. No significant change in coagulation factors or bleeding has been reported as a complication of the therapy. Hypersensitivity reactions have not been associated with the use of urokinase (but have been reported with the use of streptokinase). Irrigation of the catheter with antibiotics has not been shown to have a beneficial effect. Once a pleural peel or rind has developed (indicating a late stage in evolution of the empyema- usually several weeks) open thoracotomy and decortication are required for effective treatment. Findings which indicate that percutaneous drainage will not be effective include a honeycomb septation pattern within the empyema or a parietal pleura greater than 5 mm in thickness by sonography. [2,3]
X-ray:
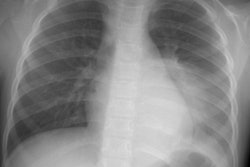
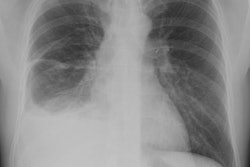
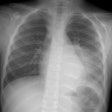
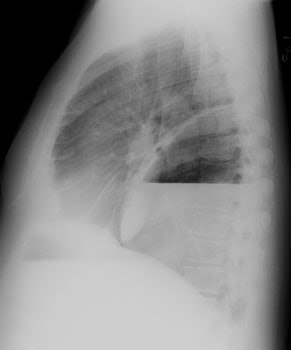
CXR: On CXR the abnormality can be localized to the pleural space because of its lenticular shape (forms obtuse angles with the chest wall, while a lung abscess is usually round with acute angles). An air-fluid level associated with an empyema is usually longer on the lateral film [7].| Empyema:
The CXR demonstrates a large, lenticular shaped air-fluid
level in the
right pleural space |
  |
Computed tomography: On CT empyemas are usually oblong,
have
a sharp interface with the lung parenchyma and displace vessels
and
bronchi
away from the empyema. Following contrast administration, there is
enhancement of the abnormally thickened parietal
and visceral pleura which are separated by the empyema ("split
pleura
sign") [6]. The split pleura sign can be seen in 68-86% of
patients
with empyema, however, it is not specific for the condition as it
can
be seen in other causes of exudative effusion as well (malignant
effusion, hemothorax, prior pleurodesis) [8,9]. Increased
density within the extrapleural fat may also be seen. The presence
of a loculated (non-dependent) pleural fluid collection in a
patient
with
a pneumonia strongly suggests the presence of an empyema. It is
important
to remember, however, that many empyemas can resemble a simple
parapneumonic
effusion. Gas bubbles in the pleural space is presumptive evidence
of a
bronchopleural fistula, but the finding may also be seen due to
infection
with a gas forming organism or following thoracentesis [4]. A
pleural
peel
appears as a markedly thickened parietal pleura which is separated
from
the chest wall by a thickened layer of extra-pleural fat. Focal or
diffuse
calcification can occur and the volume of the affected hemithorax
is
reduced.
[6]
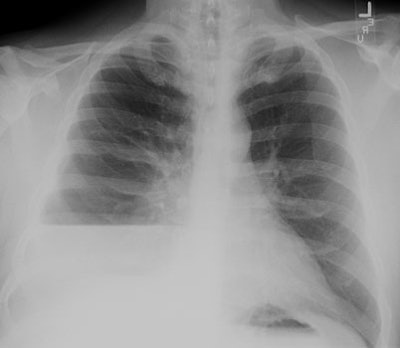
| Empyema:
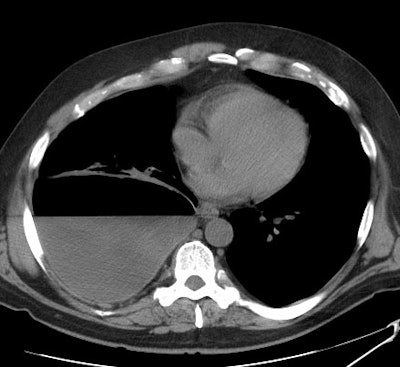
A non-contrast CT scan on the patient shown above
demonstrated a large
right pleural fluid collection with an air-fluid level.
Thickening of
the underlying pleural surface can be seen even on
non-contrast
imaging. The fluid was infected at aspiration. |
 |
REFERENCES:
(1) ACR Syllabus #40: p.483-86
(2) AJR 1996; Intracavitary urokinase therapy as an adjunct to percutaneous drainage in a patient with a multiloculated empyema. 167:643-647 (no abstract available)
(4) Radiographics 1997; Complex disease of the pleural space: the 10 questions most frequently asked of the radiologist--new approaches to their answers with CT and MR imaging. 17: 1043-1050 (no abstract available)
(6) CT/MRI Head to Toe Course Syllabus 1997; Webb WR. CT of the pleura. NYU Medical Center
(7) Radiol Clin N Am 2005; Tarver RD, et al. Radiology of community-acquired pneumonia. 43: 497-512
(8) Radiology 2007; Kraus GJ. The split pleura sign. 243: 297-298
(9) AJR 2014; Walker CN, et al. Imaging pulmonary infection:
classic signs and patterns. 202: 479-492