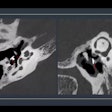
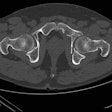
Medical imaging AI company Avicenna.AI has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its CINA-CSpine tool.
CINA-CSpine is designed to support the detection and triage of cervical spine fractures from CT images. It was validated on more than 300 noncontrast CT scans provided by multiple sources in the U.S. and Europe and acquired from 36 different scanner models across five vendors, according to the firm.
The device achieved an overall sensitivity and specificity of 90.3% and 91.9%, respectively, when compared with the ground truth established by the consensus of three U.S. board-certified senior radiologists, Avicenna added.
The company earlier in 2024 received FDA clearance for its CINA-iPE, CINA-Aspects, and CINA-VCF tools.