As the number of detector rows grows, multidetector-row CT is testing radiologists with an ever-increasing array of user-controllable acquisition parameters. With so many exposure choices available for even a single slice thickness, finding the best approach can be daunting. Yet by considering how one parameter affects the others, and how every combination affects the diagnosis and the patient, optimal diagnostic image quality can be assured.
According to Dr. Geoffrey Rubin and Dr. Dominik Fleischmann of Stanford University in Stanford, CA, the best way to approach acquisition is to answer four key questions about the scan:
- What is the thinnest section that might be useful? What are the thinnest images that might need to be reconstructed?
- How fast does the scan need to be?
- How critical is radiation dose minimization?
- To what extent might noise limit the assessment?
"I think you'll find that when you consider the individual patient scenario, one of these four issues tends to bubble to the top as being the most critical, and that's going to drive how your protocol occurs," Rubin said. Rubin and Fleischmann discussed MDCT protocol choices at the 2003 Symposium on Multidetector-Row CT in San Francisco, sponsored by Stanford University.
The question of section thickness comes down to the need to evaluate very small structures, Rubin said. When examining the victim of a motor vehicle accident, for example, detection is generally focused on larger areas of abnormality spread out over large volumes, and sections can be thicker. However, looking for pulmonary embolism requires much thinner sections, as does any application that might require 3-D visualization.
The trick is to acquire sections as thin as the thinnest slice that might need to be reconstructed to get a better look after reviewing the initial data. This approach, a staple of thoracic imaging, helps avoid the need for a second scan.
"The speed of the scan has a lot to do with the patient's ability to hold their breath ... most people do well up to about 15 seconds," Rubin said. "If we're scanning someone's leg, of course, breathholding duration is irrelevant...Contrast medium delivery is greatly impacted by the length of the scan. If we have a patient where we need to minimize contrast utilization, then we want to consider speed of scan. Also (consider) patient motion: pediatric patients or combative patients, etc., might need faster scans."
Radiation dose considerations are highly age- and organ-dependent, of course, but risk stratification matters too, Rubin said. "If a patient comes in who is severely bleeding and has severe chest pains, something that might result in their demise within 24 hours, obviously radiation exposure has a minimal impact on their overall risk relative to making the correct diagnosis," Rubin said.
As for noise, its importance generally boils down to the anticipated difference in contrast between the abnormality and the background. More noise can be tolerated in the lungs, which have inherently high contrast, and less in the liver, where intrinsic contrast is lower.
"Everything else being equal, we need to look at the anatomic region and consider what it is we're trying to detect," Rubin said. To illustrate the clinical importance of their approach, Rubin and Fleischmann presented four cases emphasizing each of the four questions in turn.
Slice thickness
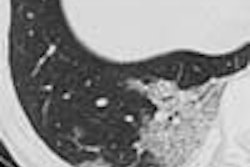
The first patient was a 40-year-old woman, post heart-lung transplant, with chest x-ray suggesting a cavitary lung nodule in the right upper lobe of a lung. The imaging goals were lesion detection with thick sections and possible lesion characterization with thin sections.
"Longitudinal spatial resolution is the key issue in this patient," Fleischmann said. "We want to see very small structures if possible, so we need narrow detector collimation. The table speed should be high."
Thus, sections 1 mm thick were acquired with a fast table speed, using 5-mm reconstructed sections for the initial search, and 1-mm reconstructions for lesion characterization. Thin-section imaging showed an airway connection, revealing a bronchial rather than a parenchymal lesion in this patient, Fleischmann said.
Scanning speed
The second patient was a 48-year-old woman with diabetes mellitus and serum creatinine of 1.7. She had undergone an axillary femoral bypass, an aortal femoral bypass, and a femoropopliteal bypass, and had contraindications to MRI, Rubin said. The diagnostic question was to assess the patency of her bypass grafts.
"Probably the primary goal is to minimize the contrast dose ... but we still need to maintain a very large volume (1,500 mm) of coverage, basically the entire chest, abdomen, pelvis, and runoff," Rubin said.
Fleischmann chose the following acquisition parameters for three different scanners:
Four-row scanner: 4 x 1.25 detector configuration, pitch 1.5, 0.5 s rotation speed, 15 mm/s table speed, reconstructed at 1.25 mm section thickness, scan time 50 seconds.
Eight-row scanner: Same as above, except 8 x 1.25 detector configuration, pitch 1.7, 34 mm/s table speed, scan time 45 seconds. Contrast injection protocol: 4 ml/s for 45 seconds = 180 ml.
Sixteen-row scanner: Same as above, except 16 x 1.25 detector configuration, pitch 1.8, 70 mm/s table speed, scan time 22 seconds. Contrast enhancement with the faster scan can be improved by either increasing the flow rate or increasing the injection, Fleischmann added.
Contrast injection protocol: 5 ml/s for 22 s = 110 ml; or increase the injection duration and the scanning delay for 8 seconds: 4 ml/s for 30 s = 120 ml.
Rubin added that when planning for contrast in even borderline azotemic patients, it is important to consider the full range of factors such as the weight of the patient, and renal protective measures such as the use of iso-osmolar contrast agents, preimaging hydration, and N-acetyl-cysteine or pharmacologic therapy, he said.
Dose considerations
Patient number three was a 37-year-old pregnant woman with shortness of breath. Her history included a left parietal brain lesion; the diagnostic goal was to rule out pulmonary embolism.
Dose was the primary concern in this patient, Rubin said, adding that it's important "to always recognize that we can shoot ourselves in the feet by reducing radiation too much and then having a non-diagnostic scan."
The scout view was eliminated completely to reduce radiation dose, and bolus tracking was used to track the contrast and then trigger the single helical acquisition. And because more detectors means less wasted radiation, use the scanner with the most detector rows available, and as high a pitch as possible, Rubin said.
"High pitch means less radiation exposure without much of an impact on image quality at all," he said. "We want to lower our mA and kV as much as we can. Here's where the art comes in, because we don't have any kind of auto-exposure control on our scanners, and we'll have to use our experience and intuition.... And we'll have to look at the size of this patient and consider the area being scanned in order to match these values to our expectations from our CT scanner."
It's important not to skimp on contrast either, since cardiac output in the third trimester of pregnancy can be as high as 150% of normal.
Thus, intravenous contrast was administered (150 ml at 5 ml per second) and images were acquired at 16 x 1.25 mm, 0.5-second rotation, 120 kVp, 100 mAs. A soft reconstruction kernel was also used to reduce noise, though at the expense of some spatial resolution. No pulmonary embolism was found, but the patient had fine micronodular abnormalities and was diagnosed with miliary tuberculosis.
Noise as a limiting factor
The last patient was a 55-year-old man with leg pain on exertion. The imaging challenge resulted from his weight: 430 lbs. at 5'10" in height.
"In some respects the principals are the same as in the last case," Rubin said, except that noise was a result of radiation reduction in the last patient, and in this case resulted from substantial attenuation in the patient's body, and radiation was less of a concern.
The contrast technique aimed at maximizing contrast dose (200 ml), maximizing iodine concentration (350 mgl/ml), and maximizing the injection rate with the aid of a 20-gauge antecubital IV. This seems like a lot of contrast, Rubin said, but less than 0.5 ml per pound means the patient is being relatively underdosed.
Images were acquired on a 16-detector scanner with collimation of 16 x 1.25 mm, maximum pitch (1.8), maximum mAs (370), and maximum rotation time of 0.7. Section thicknesses were reconstructed at 2.5 mm to minimize noise, though with a decrease in spatial resolution.
"You want to maximize the mAs product, maximize tube current and rotation time," Rubin said. "What I have found is that you're basically bumping up against tube heating; as you try to increase mAs and rotation time, the machine won't let you do that anymore." The solution is to calculate which balance between high mAs and long rotation time gives the highest mAs product for the acquisition, he said.
The resulting images were of diagnostic quality; however, no abnormality was found, Rubin said.
By Eric BarnesAuntMinnie.com staff writer
July 11, 2003
Related Reading
CT pioneer sees bright future for the technology, June 27, 2003
Single-slice MR assesses overweight youth, meaning CT could too, June 12, 2003
Gastric bypass battles bulge, but patients pose imaging challenge, March 27, 2003
Copyright © 2003 AuntMinnie.com