SAN FRANCISCO - The latest oncologic technique for treating tumors involves convention-enhanced delivery (CED). With CED, the therapeutic agent is continuously infused into the targeted area, allowing the drug to bypass the blood-brain barrier.
But determining whether CED was successful requires the development of imaging tracers for real-time, in-vivo monitoring, according to a presenter Monday at the Congress of Neurological Surgeons (CNS).
"The rationale for investigating new imaging strategies for CED is twofold," said Dr. David Croteau, who is from the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (NIH) in Bethesda, MD. Two of Croteau's co-authors, Dr. John Butman and Dr. Dennis Johnson, are from the NIH's neuroradiology division.
"Most current imaging modalities are very limited when it comes to assessing the distribution obtained by CED. More accurate methods are clearly needed to form a real-time assessment of the volume of distribution, which will allow treatment modification, if required, and provide better assessment of the given therapeutic agent," Croteau continued.
In addition, new imaging methods are required to gather real-time pharmacokinetic data, such as drug clearance, which will also guide treatment. For the study, CT was chosen because it offers adequate imaging of the supratentorial structures, Croteau said. The modality's other strengths include short exam times and readily available equipment, giving it an advantage over MRI, he added.
The animal study involved four primates that underwent CED with a total of 90-150 µL of iopamidol (Isovue, Bracco Diagnostics, Princeton, NJ). CED was performed at 0.5 µL/min for 30 to 60 minutes, then increased to 3.5 µL/min.
The researchers chose iopamidol, a low-molecular-weight tracer, "because of its favorable CNS safety profile derived from its extensive use for myelography," Croteau said.
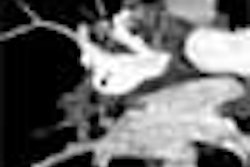
Real-time CT and postinfusion scans five days later determined the distribution of the tracer. Coronal images with a slice thickness of 1 mm were obtained, and 3D volume calculation was performed using a threshold of 10% segmentation.
The researchers also used quantitative autoradiography (QAR) with 14C-sucrose (small molecule) and 14C-dextran (large molecule), which was compared to a mathematical model. They used up to five months of clinical observation and histopathology to evaluate safety and toxicity.
Real-time CT of the tracer during infusion revealed a definable region of perfusion. The volume of distribution (Vd) increased linearly with an increased infusion volume (Vi). The overall Vd to Vi ratio was 4.1. QAR confirmed the accuracy of distribution for small (sucrose) and large (dextran) molecules, which was identifiable up to 72 hours after infusion. But the technique was more accurate for imaging small-molecule distribution, Croteau said. There was no evidence of toxicity.
In one example, he explained that the postinfusion imaging sequence was first performed immediately after infusion was completed, and showed the volume of distribution of iopamidol in the right frontal white matter. Twenty-four hours later the density of the volume decreased significantly, although there was enlargement due primarily to clearance of the agent. The tracer progressively disappeared at 48 hours, and the complete clearance was observed between 76 and 92 hours.
"CT imaging of CED using a low-molecular-weight tracer appears feasible, reliable, and safe. Pharmacokinetic data derived from these experiments reveal the linear relationship between (CED) volume distribution and volume diffusion," Croteau concluded.
An audience member asked Croteau if the manufacturer had expressed any interest in supporting an application for FDA approval for this usage of iopamidol. Croteau said his group planned to publish the results before approaching the manufacturer.
By Shalmali Pal
AuntMinnie.com staff writer
October 20, 2004
Related Reading
Transesophageal MRI monitors plaque reduction during statin therapy, October 15, 2004
CT-guided RF lung cancer ablation successful, October 13, 2004
FDG-PET offers treatment index for postchemo oral cancer, September 30, 2004
Copyright © 2004 AuntMinnie.com