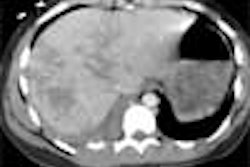
The Food and Drug Administration has given 510(k) clearance to Toshiba America Medical Systems for its SurePlaque cardiac visualization software, designed for the Aquilion CFX series of cardiac CT scanners.
The SurePlaque option identifies and quantifies soft plaque for cardiac and peripheral vessel analysis using a color-coding system based on tissue density.
By AuntMinnie.com staff writers
November 29, 2004
Related Reading
Toshiba, Thomas Jefferson start education service, November 23, 2004
Toshiba makes first U.S. Kalare install, November 22, 2004
Toshiba installs first T.Rad Plus Digital unit, November 22, 2004
Toshiba to unveil Aplio xV at RSNA show, November 19, 2004
Toshiba America Medical Systems, November 15, 2004
Copyright © 2004 AuntMinnie.com