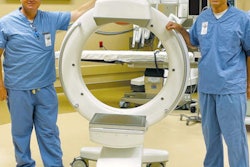
Specialty CT manufacturer Xoran Technologies of Ann Arbor, MI, has received a warning letter from the U.S. Food and Drug Administration (FDA) for documentation issues related to its MiniCAT scanner, a compact CT unit used for imaging the sinuses, skull base, and temporal bones.
In a letter dated March 25, the FDA stated that MiniCAT is "adulterated" according to the meaning of the Federal Food, Drug, and Cosmetic Act due to a number of violations of current Good Manufacturing Practices. The warning letter lists 10 specific violations, ranging from failure to maintain a complete device master record for the product to failure to document that Xoran personnel are adequately trained according to federal requirements.
The FDA acknowledges that Xoran submitted a response letter to the FDA in December 2007, outlining its intention to comply with observations noted in a previous FDA inspection. However, the agency concluded that the company's response was "inadequate" because "there are no specific steps outlined to correct the violations and prevent their recurrence."
The warning letter advises Xoran to "fully evaluate" its record-keeping system to ensure that future "deviations" do not occur, specifically regarding design history files, device master records, device history records, and product monitoring records. The agency asks Xoran to submit a response within 15 working days of the receipt of the warning letter, detailing the steps the company plans to take to address the deficiencies.
In a written statement to AuntMinnie.com, Xoran spokesperson Susie Vestevich said there were "no findings of safety violations, and there will be no interruption in the production, sales, or service of our products."
Xoran "stand(s) behind the quality and safety of our products, and we are committed at all times to meeting or exceeding the thresholds for safety and efficacy set out by the FDA, ISO (International Organization for Standards), and all other applicable regulatory agencies," she added.
Through its regular audits, the FDA "invariably will identify areas for improvement to ensure full technical compliance with the regulations," Vestevich stated. "Xoran has recently undergone such an audit, where the FDA identified some areas for improvement, primarily related to supplementing the documentation of our FDA files."
Related Reading
Road to RSNA, CT, Xoran Technologies, October 22, 2007
Varian, Xoran in DR supply deal, March 21, 2005
Copyright © 2008 AuntMinnie.com