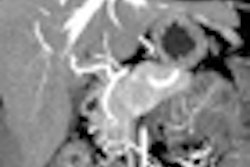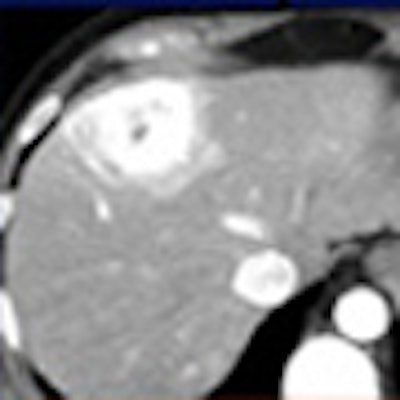
LAS VEGAS - Dual-energy CT using different tube energy (kVp) settings can be used to distinguish imaging targets with similar densities but different attenuation properties -- iodine contrast and water, for example -- thereby increasing the conspicuity of contrast-enhanced lesions.
The concept of dual-energy imaging is hotter than the Nevada sun at this year's International Symposium on Multidetector-Row CT, where manufacturers are showing off their scanners' capabilities for leveraging different material absorption properties to improve CT's diagnostic capabilities.
You don't need a dual-energy CT scanner for selective iodine imaging, though you will need a high-output x-ray tube on your regular CT scanner, according to a presentation at Wednesday's abdominal sessions.
Dr. Rendon Nelson of the Duke University School of Medicine in Durham, NC, discussed how hypervascular liver lesions can be detected more confidently and in greater numbers by dropping the kVp setting and increasing mAs, based on research conducted at Duke. The study moves a 1970s proof of concept (Medical Physics, November 1977, Vol. 4, pp. 474-481) into current clinical practice.
"Use low kV to increase the conspicuity of lesions that hyperenhance since (low) kV is closer to the K-edge of iodine, then use high mAs to decrease the noise and make the images readable," said Nelson, who is a professor and vice chair of radiology at Duke.
K-edge refers to a sudden increase in the attenuation coefficient of photons due to photoelectron absorption. It occurs at a photon energy level just above the binding energy K-shell electron of the atoms interacting with the photons, or 33.2 kev (kilovolt estimated valuation) in the case of iodine. With CT, this is the energy level at which iodine is brightest compared to other materials.
Selective enhancement techniques might even help CT regain some of the ground it has lost to MRI in hepatic imaging. While CT does a better job of defining liver vasculature compared to MRI, visualization of small, subtly enhancing lesions is unreliable. The most frequently missed lesions include primary tumors in the cirrhotic liver and metastatic endocrine tumors.
"We still miss primary lesions, maybe up to 30% of lesions that are not well seen in existing state of the art and even MR. We also miss some metastatic lesions, though I suspect there's a smaller number compared to (hepatocellular carcinoma)," he said.
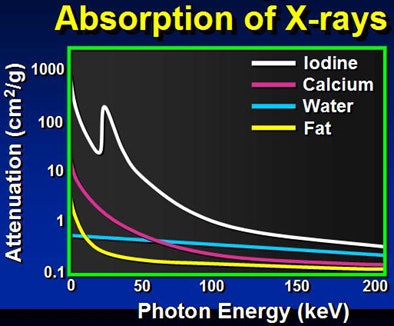
Because low kVp is closer to the K-edge of iodine, the attenuation of iodine structures increases as kVp decreases. Thus, a CT image of an iodine structure imaged at 140 kVp is about 200; dropping the kVp to 80 nearly doubles the attenuation, even though tube energy is reduced, Nelson explained.
In a phantom model tested by the Duke team, iodine structures were seen more clearly when attenuation was reduced. Similar results were found in patients with hepatic lesions; some subtly hyperenhancing liver lesions could be seen only at lower kVp levels.
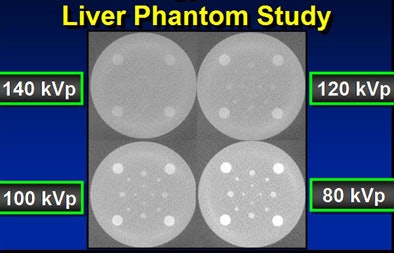
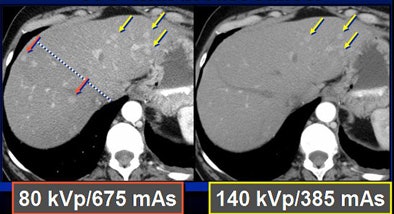
To test the hypothesis quantitatively, Duke researchers performed a study of 48 patients with 68 hyperenhancing live lesions, scanned using a dual-energy volume technique (GE Healthcare, Chalfont St. Giles, U.K.) during the arterial phase, Nelson said. One rotation was scanned at 80 kVp and 540 mAs; a second was acquired at 140 kVp and 308 mAs. The results showed significant differences in lesion conspicuity with the use of low-kVp imaging.
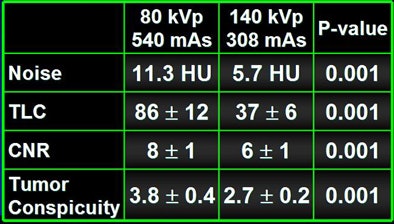
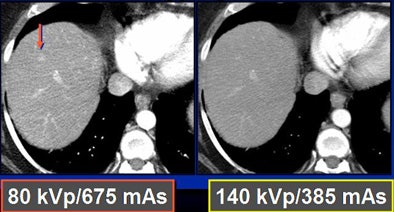
Dropping kVp increases image noise substantially, he said. "When you cut the kVp, you drop your radiation dose to one-fourth, but you increase your noise by a factor of four using roughly the same mAs," Nelson said. By increasing the mAs to compensate, "you increase the noise by about a factor of two and decrease the dose by about a factor of two by going from 140 kVp to 80 kVp."
The only equipment requirement is an x-ray tube capable of 600 mAs or higher output on your CT scanner; dual-energy hardware and software are unnecessary, Nelson said. The researchers propose the use of lower kVp during the arterial phase but standard high kVp during the portal venous phase.
The most salient trade-off is increased image noise with lower kVp; however, techniques in development, such as adaptive statistical iterative reconstruction (ASIR), can help improve the appearance of the noisy images, he said.
"The technique has excellent potential during the hepatic arterial phase for increasing the number and confidence of lesion detection," Nelson said.
By Eric Barnes
AuntMinnie.com staff writer
May 15, 2008
Related Reading
CT maintains edge over MRI in liver transplant planning, March 10, 2008
At-risk liver cancer patients should get US screening, February 12, 2008
Advanced liver imaging shapes surgical strategies, September 18, 2007
Liver stiffness tied to portal hypertension in cirrhosis, May 16, 2007
Elastography noninvasively assesses hepatic fibrosis in HIV/HVC patients, February 7, 2006
Copyright © 2008 AuntMinnie.com