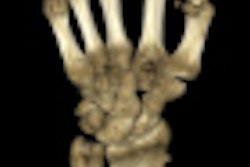
Finnish imaging vendor Planmed said it has received U.S. Food and Drug Administration (FDA) clearance for its Verity extremity scanner.
The CT scanner is intended for use on both upper and lower extremities, offering fast 3D imaging at the point of care, Planmed said. It is designed to detect subtle extremity fractures that are often missed using 2D radiographs, and it can be used both before and after surgery, delivering higher resolution at a lower radiation dose than full-body CT. The scanner also permits weight-bearing imaging of the extremities, Planmed said.
The scanner adapts to the patient with anatomy-specific imaging programs, movements, and positioning trays, the company said. For the operator, the scanner features adjustable interfaces and efficient workflow settings. The scanner previously received the CE Mark and is available in the European Union.