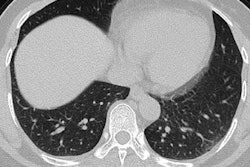
The U.S. Food and Drug Administration (FDA) has cleared Fluidda's functional respiratory imaging (FRI) online platform.
Broncholab provides a number of FRI parameters to physicians to assist in the diagnosis and monitoring of respiratory diseases and to advance personalized medicine. FRI combines high-resolution CT scans, computational fluid dynamics, and artificial intelligence with the goal of reducing clinical trials to make them more cost-effective.
"We are living in a time where respiratory viruses cause significant disruption to daily life with high associated cost," said Jan De Backer, PhD, Fluidda CEO, in a statement. "We are striving to better understand respiratory illnesses with our novel technology to be more prepared for the next viral outbreak and to deal with the increased number of patients with lung diseases worldwide."