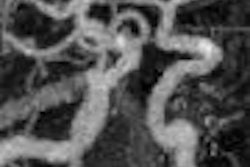
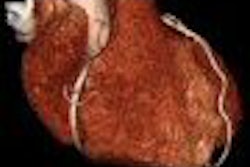
Coronary imaging developer InfraReDx of Burlington, MA, has received marketing clearance from the U.S. Food and Drug Administration (FDA) for its LipiScan NIR (near-infrared) catheter imaging system.
LipiScan is cleared for cardiac angiography, as physicians evaluate patients with symptoms of coronary heart disease.
LipiScan uses infrared imaging to detect lipid core-containing plaques and assesses a patient's coronary artery lipid core burden index. The device places a catheter equipped with a fiber-optic laser light into the artery and shines the near-infrared light through the blood to the artery wall to measure the light reflected back from the artery wall.
During this spectroscopy procedure, reflected wavelengths vary depending on how much fat and other substances are in the plaque in the illuminated portion of the wall.
Related Reading
InfraReDx receives FDA clearance, October 19, 2006
Near-infrared spectroscopy can identify vulnerable plaques in atherosclerosis, September 29, 2002
Copyright © 2008 AuntMinnie.com