Paris, France-based imaging software developer Milvue has received clearance from the U.S. Food and Drug Administration (FDA) for its TechCare Trauma AI algorithm.
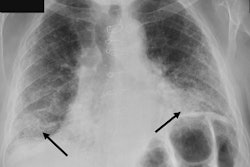
 TechCare Trauma. Image courtesy of Milvue.
TechCare Trauma. Image courtesy of Milvue.
The software enables real-time detection and localization of fractures and elbow joint effusions on x-rays. TechCare Trauma is designed for use in both adults and children and was validated in a study that included more than 7,000 U.S. patients, the company said. It has demonstrated high accuracy and consistent performance across different anatomical regions, imaging views, patient demographics (age and sex), imaging hardware manufacturers, and in both displaced and nondisplaced fractures, according to the vendor.
TechCare Trauma is available via a secure cloud-based platform or on-premises deployment, Milvue noted.