Testing patients for how they respond to blood thinners via platelet function tests (PFTs) before undergoing treatments for brain aneurysms can reduce their chances of subsequent stroke, according to a study published March 18 in Radiology.
The finding is from a clinical trial in which half of the patients received the tests prior to procedures and half did not, noted lead authors Yangyang Zhou and Jun Wang, of Capital Medical University in Beijing, China, and colleagues.
“PFT-guided antiplatelet therapy can reduce the incidence of [ischemic events] compared with [standard dual antiplatelet therapy] in patients undergoing endovascular intervention for [intracranial aneurysms],” the group wrote.
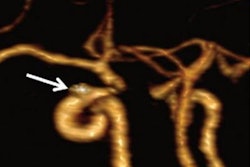
Patients undergoing stent placements to treat brain aneurysms typically receive dual antiplatelet therapy (aspirin and clopidogrel) to thin their blood prior to procedures. This reduces the formation of blood clots after procedures, which can lead to strokes, the authors explained.
However, patients differ in how they respond to antiplatelet therapy, with some patients more prone to clotting, they noted. PFTs prior to treatment can indicate whether patients are responding to the drugs, yet to date, there have been no clinical trials to test whether PFTs improve outcomes, the authors wrote.
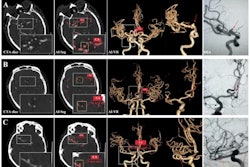
Thus, in a prospective trial in 590 participants (median age, 58 years old), the researchers tested whether one such test, a light transmission aggregometry test (AG800, Techlink Biomedical Technology), could reduce strokes in patients after stent placements. The trial involved sixteen treatment teams from eight public hospitals across three cities between May and August 2023.
The researchers split the group in half. In one group, participants who showed poor antiplatelet response after the test were administered an increased aspirin dose or were switched from clopidogrel to ticagrelor. The control group underwent no test and were administered standard dual antiplatelet therapy (SDAT). The primary outcome was any cerebral ischemic event within 30 days after the procedure.
According to the analysis, ischemic events were lower in the test group than in the control group (6.8% [20 of 295] vs. 13.2% [39 of 295]; p = 0.03) after undergoing the procedure, with no evidence of a difference between the groups in modified Rankin Scale scores (disability scale scores).
Furthermore, there was no evidence of a between-group difference in bleeding events (24.1% [71 of 295] in the test group vs. 31.2% [92 of 295]; p = 0.32), the group noted. This was significant, given that ticagrelor can increase the risk of bleeding, the authors noted.
“For participants with intracranial aneurysm who underwent stent treatment, light transmission aggregation-guided antiplatelet therapy helped reduce ischemic events without increasing bleeding risks,” the researchers wrote.
In an accompanying editorial, David Kallmes, MD, of the Mayo Clinic in Rochester, MN, and Dorothea Altschul, MD, of the Valley Hospital in Paramus, NJ, noted that the multicenter trial corroborates outcomes from previous, smaller, single-center studies and "without question" moves the field forward.
Yet they noted limitations, primarily that the results may be difficult to generalize to other centers. The PFT used in the trial (light transmission aggregometry) is only one of many assays, which also include platelet aggregometry with multiple possible agonists and thromboelastography, for instance.
“Research is sedimentary, with layers upon layers of incremental advances adding up to profound improvements over time. Future studies would be well served to reflect on this important study,” Kallmes and Altschul concluded.
The complete study can be found here.