CHICAGO - A new theranostics imaging and treatment approach shows promise in patients with pancreatic cancer, according to research presented at the Society for Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
A group at University of California, Davis performed a first-in-human study to evaluate the safety of the theranostic peptide pair gallium-68 (Ga-68) DOTA-5G (imaging tracer) and lutetium-177 (Lu-177) DOTA-ABM-5G (radiotherapeutic) in patients with locally advanced or metastatic pancreatic adenocarcinoma.
"Our goal with this study was to develop a theranostic strategy to facilitate both earlier detection and more effective treatment for this devastating malignancy," said Julie Sutcliffe, PhD, in a June 24 presentation.
The American Cancer Society estimates that more than 64,000 people will be diagnosed with pancreatic cancer in 2023, and more than 50,000 of them will die from the disease -- a 5-year survival rate of only 12%.
Theranostics refers to the dual approach of using radiopharmaceuticals to both identify and treat cancer and is an emerging field in nuclear medicine. Theranostics may be described as, "We see what we treat, and we treat what we see," Sutcliffe said. The first treatment in this class approved by the U.S. Food and Drug Administration was Pluvicto in March 2022.
Integrin alpha-v beta-6 is a cell surface receptor overexpressed by epithelial cells in pancreatic cancer tumors. Ga-68 DOTA-5G is an integrin alpha v beta 6-targeted peptide that shows promise as a diagnostic imaging agent, while Lu-177 DOTA-ABM-5G contains the same integrin alpha v beta 6-targeted peptide and has shown promise as a radiotherapeutic.
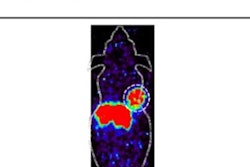
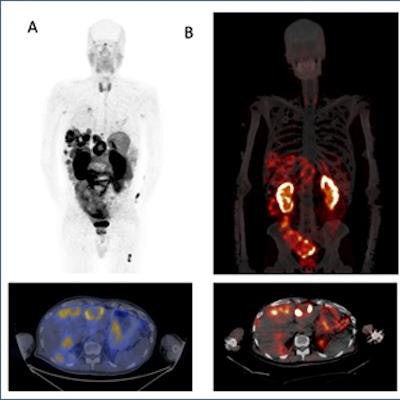
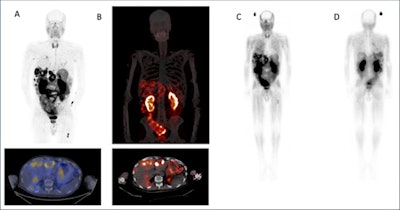 (A) Ga-68 DOTA-5G PET/CT images (1 hour post injection). (B) Lu-177 DOTA-ABM-5G SPECT/CT images (1 day post injection). (C) Lu-177 DOTA-ABM-5G whole body planar image (anterior, 1 day post injection). (D) Lu-177 DOTA-ABM-5G whole body planar image (posterior, 1 day post injection). Image courtesy of Julie Sutcliffe, PhD.
(A) Ga-68 DOTA-5G PET/CT images (1 hour post injection). (B) Lu-177 DOTA-ABM-5G SPECT/CT images (1 day post injection). (C) Lu-177 DOTA-ABM-5G whole body planar image (anterior, 1 day post injection). (D) Lu-177 DOTA-ABM-5G whole body planar image (posterior, 1 day post injection). Image courtesy of Julie Sutcliffe, PhD.To date, 17 patients have been imaged with Ga-68 DOTA-5G PET/CT and 14 patients have been treated with Lu-177 DOTA-ABM-5G, Sutcliffe said. The primary objective was reached -- both agents were well tolerated, and no drug-related serious adverse events were observed, she said.
Additionally, Ga-68 DOTA-5G PET/CT imaging was able to detect bone, lung and hepatic metastasis, and the Lu-177 DOTA-ABM-5G radiotherapeutic was taken up and retained by the tumors detected by the tracer.
The results will pave the way for additional clinical trials in patients with other malignancies including but not limited to non-small cell lung cancer, breast cancer, and head and neck cancer, Sutcliffe said.
"This approach has the potential to significantly improve the clinical care and treatment for patients with metastatic disease," she said.
The phase I arm of the study will be completed in the summer of 2023, and researchers anticipate that a second trial in patients with metastatic non-small cell lung cancer will open to enrollment in fall 2023. A third trial for patients with metastatic carcinoma of any primary origin is expected to commence in the winter of 2023, Sutcliffe noted.