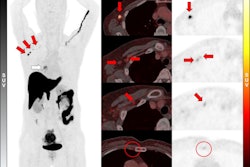
Adding F-18 fluroestradiol (FES)-PET/CT imaging to standard of care tests in women with suspected estrogen receptor (ER)-positive breast cancer could save the U.S. millions in healthcare costs, according to a study published May 14 in PLOS One.
In a budget impact study, GE HealthCare scientists estimated the approach could save up to $142 million over a five-year period, primarily by increasing true-positive and true-negative test results and reducing the need for biopsies, noted a team led by Regina Munter-Young, the company’s head of global market access.
“Adding F-18 FES-PET/CT to biopsy/[immunohistochemistry] may increase the diagnostic accuracy of the ER status, especially when a tumor sample cannot be obtained, or the risk of a biopsy-related complication is high,” the group wrote.
Around 70% of breast cancers express estrogen receptors. Correct identification of ER status is crucial to optimize treatment, yet standard of care, involving biopsy and immunohistochemistry (IHC), and other diagnostic tools such as F-18 FDG-PET can yield inconclusive results, the group explained.
F-18 FES-PET was approved in May 2020 for detecting ER-positive lesions in patients with recurrent or metastatic breast cancer. The radiotracer was developed by French biomarker firm Zionexa, which GE HealthCare acquired in May 2021.
To date, only a limited number of economic analyses have been performed for F-18 FES-PET/CT, the authors noted.
To address this knowledge gap, the researchers culled data from studies in women with breast cancer published between January 1980 and May 2020 and compared costs between two scenarios: Scenario A, where ER-positive status was determined solely through biopsy/IHC and scenario B, where F-18 FES-PET/CT was used in addition to biopsy/IHC.
To establish the total cost per scenario, the model considered the cost of the molecular imaging tracer and any diagnostic imaging scans, the cost of biopsies, treatment costs, costs of managing treatment-related adverse events (TRAEs), and other relevant categories of cost, such as end-of-life care.
The cost comparison assumed that F-18 FES-PET/CT could accurately classify the ER status in three patient groups: metastatic breast cancer patients when biopsies failed or were inconclusive, metastatic breast cancer patients when biopsies were not possible, and in all recurrent breast cancer patients.
According to the results, the addition of F-18 FES-PET/CT to biopsy/IHC (Scenario B) would increase the proportion of true-positive and true-negative test results from 0.2% to 8% compared to the sole use of biopsy/IHC (Scenario A), while reducing re-biopsies by 94% to 100%.
Overall, the researchers thus estimated that adding F-18 FES-PET/CT to biopsy/IHC would result in a five-year cost savings of up to $142 million.
“The advantage of FES is particularly evident when a tumor sample cannot be obtained, or the risk of a biopsy-related complication is high,” the group wrote.
Ultimately, there is a growing body of evidence that questions the ability of conventional diagnostics and imaging to detect distant disease in low-metabolic diseases in women with breast cancer, with appropriate ER status being essential to inform therapeutic plans, the group wrote.
“Oncological centers can benefit from using F-18 FES-PET/CT as it not only increases confidence in their actions but can also result in cost savings,” the researchers concluded.
The full study can be found here.