PET scans that determine whether men with prostate cancer are eligible for Pluvicto treatment can provide additional clues on how they may respond to the drug, according to a study published August 20 in Radiology.
In an analysis of clinical trial data, researchers found that uptake by tumors of the radiotracer gallium-68 (Ga-68) prostate-specific membrane antigen (PSMA)-11 was strongly associated with improved outcomes.
“We found that the whole-body tumor mean standardized uptake value (SUVmean) was the best predictor of Lu-177 [lutetium-177] PSMA-617 [Pluvicto] efficacy for all outcomes tested,” wrote lead authors Phillip Kuo, MD, PhD, of the City of Hope in Duarte, CA, and Michael Morris, MD, of Memorial Sloan-Kettering Cancer Center in New York City.
Ga-68 PSMA-11 PET scans were used to identify candidates for Pluvicto clinical trials and are currently used to determine whether men are eligible for the treatment (Pluvicto was approved on March 22), the authors explained.
Clinicians visually interpret these scans, yet research suggests that analyzing quantitative PET data can provide additional information, such as how effective the drug may be on a per-patient basis.
To explore the issue, the researchers analyzed baseline Ga-68 PSMA-11 PET scans of 826 participants from the VISION trial, a phase-III trial conducted between June 2018 and October 2019 to determine Pluvicto’s benefit. All participants had pretreated metastatic castration-resistant prostate cancer.
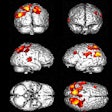
 Whole-body anterior coronal PSMA-PET maximum intensity projection images in a 63-year-old white male participant with tumors in the liver, bone, and lymph node (LN) who had an initial prostate-specific antigen level of 181.9 ng/mL and Eastern Cooperative Oncology Group performance score of 0/1. The images show all PSMA-positive (PSMA+) disease as a single whole-body volume in red (left) and segmented according to anatomic region (right; bone lesions in blue, liver lesions in green, lymph node lesions in red).Image and caption courtesy of RSNA
Whole-body anterior coronal PSMA-PET maximum intensity projection images in a 63-year-old white male participant with tumors in the liver, bone, and lymph node (LN) who had an initial prostate-specific antigen level of 181.9 ng/mL and Eastern Cooperative Oncology Group performance score of 0/1. The images show all PSMA-positive (PSMA+) disease as a single whole-body volume in red (left) and segmented according to anatomic region (right; bone lesions in blue, liver lesions in green, lymph node lesions in red).Image and caption courtesy of RSNA
Key findings included the following:
- Baseline Ga-68 PSMA-11 PET SUVmean was strongly associated with improved outcomes following Pluvicto therapy versus controls (hazard ratio [HR] range, 0.86–1.43; p < 0.001)
- A one-unit whole-body tumor SUVmean increase was associated with a 12% decrease in the risk of progression-free survival and a 10% decrease in the risk of death.
- A higher PSMA-positive tumor volume was associated with worse overall survival (HR range, 1.38–2.12; p ≤ 0.006)
“High SUVmean values indicate high average PSMA expression levels, which may promote tumor binding and uptake of Lu-177 PSMA-617, leading to greater delivery of radiation and greater antitumor activity,” the group wrote.
Ultimately, using quantitative PET analysis for patient selection is difficult in clinical practice, as SUV measurements outside of clinical trials are subject to variability, the researchers noted. This is due to technical factors such as the PET scanners and acquisition and reconstruction parameters.
Further research could help standardize whole-body tumor SUVmean quantification using automated or AI software, they suggested.
“Therefore, SUVmean may have a role in patient selection in the future,” the researchers concluded.
The full study is available here.