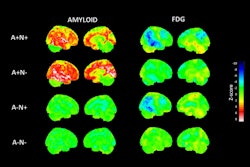
Amyloid PET scans in individuals with early signs of Alzheimer’s disease can identify those who are at higher risk of progressing to cognitive decline, researchers in Germany have reported.
The finding is from an analysis of 197 individuals with “subjective cognitive decline” and highlights the value of PET scans for identifying people with pre-clinical disease, noted lead author Guilherme Kolinger, MD, of Life Molecular Imaging in Berlin, and colleagues.
“These findings suggest a promising approach for detecting pre-clinical Alzheimer’s disease within a population experiencing subjective cognitive decline, which may aid in the appropriate selection of individuals for effective interventions,” the group wrote. The study was published April 23 in the European Journal of Nuclear Medicine and Molecular Imaging.
Subjective cognitive decline (SCD) refers to the self-perception of cognitive decline that is not yet captured on standard cognitive tests. Compared to the general population, individuals with SCD have a higher risk of developing dementia. Therefore, the SCD population provides an early window to study and understand Alzheimer’s disease before the brain is irreversibly damaged, the researchers explained.
To explore the predictive value of PET in these patients, the researchers gathered data from 197 individuals with SCD who participated in a large clinical trial in Spain and who had baseline F-18 florbetaben (Neuraceq, Life Molecular) PET scans and several follow-up scans over a five-year period.
The researchers categorized individuals based on baseline Centiloid values (CL) into amyloid positive (CL > 35.7), “gray zone” (20 < CL ≤ 35.7), and amyloid negative (CL ≤ 20) groups. They further split the amyloid negative group into two groups: N1 (CL ≤ 13.5) and N2 (13.5 < CL ≤ 20). Centiloids are voxel (3D pixel) measurements that indicate areas with amyloid deposits, with values based on a 100-point scale.
 Volume with higher baseline signal for N1 accumulators and N1 non-accumulators of amyloid (red line) overlayed on the scan of an N1 accumulator (MNI space). Grey scale shows SUVR range. Blue line shows the Centiloid target region and the green line shows the reference region (whole cerebellum). Image and caption available for republishing under Creative Commons license (CC BY 4.0 DEED, Attribution 4.0 International) and courtesy of the European Journal of Nuclear Medicine and Molecular Imaging.
Volume with higher baseline signal for N1 accumulators and N1 non-accumulators of amyloid (red line) overlayed on the scan of an N1 accumulator (MNI space). Grey scale shows SUVR range. Blue line shows the Centiloid target region and the green line shows the reference region (whole cerebellum). Image and caption available for republishing under Creative Commons license (CC BY 4.0 DEED, Attribution 4.0 International) and courtesy of the European Journal of Nuclear Medicine and Molecular Imaging.
According to the analysis, at baseline, 20 individuals were amyloid positive, eight were classified as gray zone, 160 as N1, and nine as N2. Individuals with higher CL levels had an increased risk over time of converting to mild cognitive impairment (MCI).
Over five years, 19% of N1 individuals were amyloid accumulators, despite very low amyloid levels at baseline. Meanwhile, 89% of the N2 group accumulated amyloid, as well as all gray zone individuals (which had the highest rate of amyloid accumulation, 5.1 CL/year).
Finally, in a parametric image analysis of N1 accumulators, a region within the precuneus was linked to increased amyloid over time, the researchers reported.
“This finding could support very early indication of SCD individuals being at increased risk of future cognitive decline, especially because higher amyloid loads increase the chance of conversion to [mild cognitive impairment],” the researchers wrote.
Ultimately, however, it remains uncertain if the cognitive decline observed in all participants who converted to mild cognitive impairment is related to Alzheimer’s disease, as mild cognitive impairment is a clinical diagnosis with several potential underlying causes, the researchers noted.
Nonetheless, the study also validates that the precuneus is a cortical region with early amyloid accumulation in Alzheimer’s disease and that its focal signal can lead to positive visual PET reads on images from patients with low CL values, the group added.
“The presented results show that even at a very low [beta amyloid] burden this pattern could be recognized, highlighting the power of amyloid PET quantification in detecting very early signs of amyloid pathology in the brain and supporting existing evidence of early brain changes due to [beta amyloid] burden,” the researchers concluded.
The full study can be found here.