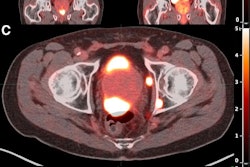
Pluvicto has met its primary endpoint in a phase III trial testing the treatment in men with prostate-specific membrane antigen (PSMA)-positive metastatic hormone-sensitive prostate cancer (mHSPC).
The treatment showed statistically significant and clinically meaningful benefit when used in combination with hormone therapy versus hormone therapy alone, with a positive trend in overall survival for patients, Novartis said in a statement.
The findings are from an interim analysis of the open-label, prospective PSMAddition trial in which participants are receiving either Pluvicto in combination with standard of care (androgen receptor pathway inhibitor therapy and androgen deprivation therapy) or standard of care alone.
“Results from PSMAddition in mHSPC show potential for treatment in an earlier setting with Pluvicto,” the company noted.
Pluvicto (lutetium-177 PSMA-617) was first approved in 2022 for adult patients with PSMA-positive metastatic castration-resistant prostate cancer who have previously received standard of care treatments. In May, it was approved for use after one androgen receptor pathway inhibitor (ARPI) treatment as well as prior to chemotherapy.
Data on this latest finding will be presented at an upcoming medical meeting and, based on U.S. Food and Drug Administration feedback, will be submitted for regulatory review in the second half of the year, Novartis noted.