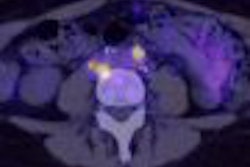
PharmaLogic P.E.T. Services of Montreal has received approval from the Canadian government to begin selling its formulation of FDG.
Health Canada has issued a notice of compliance (NOC) to the Lachine, Quebec, company. The radiopharmaceutical is indicated in patients with known or suspected abnormalities found by other testing modalities in the assessment of abnormal glucose metabolism to assist in characterization of single pulmonary nodules, staging of lung cancer, detection of recurrence in patients with previously diagnosed lung cancer, and monitoring of the therapeutic response in lung cancer patients.
Copyright © 2008 AuntMinnie.com