(Booth 4058) Healthcare IT and advanced visualization developer Merge Healthcare of Milwaukee brings its Cedara I-Response application, designed to support clinical trial workflow and oncology viewing, to Chicago this year.
I-Response supports double-blind reading, image registration, advanced segmentation, comparison of multiple time points, and support for clinical trial rules.
- The base Lesion Analysis package, which streamlines the clinical trial workflow of creating/exporting the response evaluation criteria in solid tumors (RECIST), World Health Organization (WHO) criteria, and volume measurements
- The PET/CT Analysis package, which enables users to compare standardized uptake values (SUV) over multiple time points
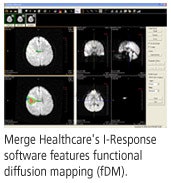
- The Functional Diffusion Mapping (fDM) Analysis package, which tracks changes in tumors at the metabolic or cellular level over multiple time points; fDM is a patented MR tumor analysis technique
Merge expects I-Response to be available in the fourth quarter, and the software has received U.S. Food and Drug Administration 510(k) clearance. Oncology, clinical trials, drug development, and PACS will be among the software's target markets.