Multimodality vendor Siemens Healthcare of Malvern, PA, plans to take the next step into hybrid imaging with the launch of Biograph mMR, a new integrated PET/MRI scanner, later this month at the RSNA meeting in Chicago.
The work-in-progress scanner is a fully integrated, whole-body PET/MR system that features simultaneous data acquisition technology and a specially developed and designed PET detector array with lutetium oxyorthosilicate (LSO) crystals that fits inside the MRI.
 |
| Biograph mMR PET/MRI. Image courtesy of Siemens Healthcare. |
With simultaneous acquisition by both modalities, a fused dataset is available automatically, but, at the same time, both datasets can be viewed separately. "For the first time, it is possible to do an assessment of oncologic disease in the head and brain, and the whole body," Maerzendorfer said.
Technical challenges
Siemens and other imaging vendors are hoping that PET/MRI follows the same successful trajectory as another hybrid imaging modality, PET/CT. The first hybrid PET/CT scanner was demonstrated by SMV America at the 1999 RSNA show, and the modality went on to almost totally replace conventional PET in developed countries, as imaging specialists recognized the value in melding the anatomical information found in CT with the functional data revealed by PET.
PET/MRI has proved to be a somewhat tougher nut to crack, however. In a PET/MRI scanner, the PET detector must function inside an unfriendly environment of high magnetic and radiofrequency (RF) fields.
"With normal detector technology, you cannot get a signal. So, therefore, it was necessary to develop a completely new, state-of-the-art PET detector technology with completely new cooling," said Britta Fuenfstueck, Siemens' CEO for molecular imaging. The PET detector "is integrated into the MR in such a way that you can do isocentric scanning and you are looking into the same field-of-view at the same time with the MR and the PET."
In addition to Siemens, Philips Healthcare of Andover, MA, has publicly acknowledged its development of a PET/MRI system. At the 2010 European Congress of Radiology (ECR), the company discussed its work on a system that employs separate PET and MRI gantries, with the patient shuttled between the two systems and images fused following data acquisition.
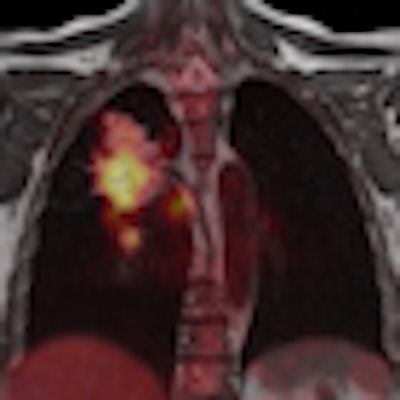
 |
| PET/MRI images courtesy of Siemens Healthcare. |
The roots of the Biograph mMR project go back a couple of years ago, when Siemens' MR and PET divisions collaborated on a prototype scanner called the BrainPET. Researchers developed the prototype for eventual use with a 3-tesla MRI system. At that time, the device had a 60-cm bore, and its only clinical application was for brain imaging.
Siemens installed four BrainPET prototypes about two years ago, Fuenfstueck said, with two sites in Germany, one device at Emory University in Atlanta, and one unit at Massachusetts General Hospital in Boston. "From these prototypes, we acquired significant insight of how to further optimize the system," she added.
The first installation of the Biograph mMR is taking place this month at University Hospital, Klinikum rechts der Isar, at Munich Technical University in Germany. Siemens expects to show clinical images from the first installation at the upcoming RSNA conference.
"Neurology is one of the key clinical areas where the system suggests significantly new insights from a medical perspective," Fuenfstueck said.
Maerzendorfer also mentioned Biograph mMR's use with both current and future PET tracers. "The classical PET tracers that are available today can combine the outstanding soft-tissue contrast of MR with the physiological function and capabilities of PET," he said. "We also see neurodegenerative diseases as another field. I would also mention pediatric applications, where CT is not used on children to reduce their exposure levels to radiation."
Future plans
Asked if the specially designed PET detector may be of use in other Siemens' technology or expanded into future products, Fuenfstueck did not think that was a possibility. She added that the detector is "a very expensive technology compared to PET detectors already in a PET/CT. ... We will not use this technology on the PET/CT side."
After its RSNA debut, Maerzendorfer said Siemens will move forward with the Biograph mMR, as it would with any other new product or technology. The company plans to have a formal release in Europe in the second half of 2011, while in the U.S., the company will submit the system through the 510(k) process.
The company's goal is to have a 510(k) application with the U.S. Food and Drug Administration (FDA) pending before the end of 2011, with the company's long-term goal to have Biograph mMR commercially available worldwide by 2012.
By Wayne Forrest
AuntMinnie.com staff writer
November 19, 2010
Related Reading
Siemens wins U.K. PET/CT contract, November 17, 2010
Charges hurt Siemens Healthcare Q4 results, November 15, 2010
Siemens partners with ScImage, November 15, 2010
Siemens offers PERCIST tool for oncology, November 3, 2010
Siemens celebrates CT milestone, November 2, 2010
Copyright © 2010 AuntMinnie.com




















